Back
Poster Session D
Session: (2154–2178) Systemic Sclerosis and Related Disorders – Clinical Poster III
2164: Profibrotic Alveolar Macrophages as a Potential Biomarker in Systemic Sclerosis-associated Interstitial Lung Disease
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Monique Hinchcliff, MD, MS
Yale School of Medicine
Westport, CT, United States
Abstract Poster Presenter(s)
Nikolay Markov1, Karolina Senkow1, Anthony Esposito2, Jonathan Puchalski3, Mridu Gulati3, Erica Herzog3, Danielle Antin-Ozerkis4, Mary Carns5, Alyssa Williams6, Nic Page6, Alexander Misharin1 and Monique Hinchcliff7, 1Northwestern University Feinberg School of Medicine, Chicago, IL, 2Brigham and Women's Hospital, Department of Medicine, Boston, MA, 3Yale School of Medicine, New Haven, CT, 4Section of Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, New Haven, CT, 5Northwestern University Division of Rheumatology, Chicago, IL, 6Yale University, New Haven, CT, 7Yale School of Medicine, Westport, CT
Background/Purpose: Interstitial lung disease (ILD) is a leading cause of death in patients with systemic sclerosis (SSc). Previously, profibrotic monocyte-derived alveolar macrophages (MoAM) expressing (SPP1, MMP9, CHIT1, CHI3L1) have been shown to play a causal role in animal models of pulmonary fibrosis and were expanded in the lungs of SSc patients with lung fibrosis undergoing lung transplantation. We hypothesized that pro-fibrotic MoAM will be also present in SSc-ILD patients without end-stage fibrosis, and that their abundance and gene expression levels will correlate with a decline in lung function.
Methods: Nine patients with SSc and 5 healthy controls (HC) were prospectively recruited at two academic medical centers to undergo bronchoalveolar lavage (BAL). Single-cell transcriptomic (scRNA-seq) analysis of isolated immune cells was performed, and data were integrated with previously published data from 10 HC from Mould et al., 2020. Confirmatory immunohistochemistry and sm-FISH staining for macrophage subtype markers were conducted in SSc-ILD lung explants and donor lungs. Numbers of pro-fibrotic MoAM were compared to pulmonary function tests (PFT) results using Pearson correlation.
Results: Integrative analysis of scRNA-seq data of BAL samples from 9 SSc-ILD patients, 15 HC identified distinct transcriptional subsets of tissue-resident alveolar macrophages (TRAM) and MoAM. MoAM were also present in HC but were more abundant in SSc-ILD patients. Two MoAM subpopulations were characterized by expression of genes (SPP1, MMP9, CHIT1, CHI3L1) causally associated with pulmonary fibrosis. Moreover, the abundance of MoAM subpopulations in BAL from patients with SSc showed significant inverse correlation (r=−0.802±0.048) with lung function as measured by forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). Staining SSc-ILD lung explant sections for markers of profibrotic MoAM subpopulations revealed that pro-fibrotic macrophages co-exist within the same alveoli with non-fibrotic macrophages defined by lack of expression of SPP1, MMP9, CHIT1, CHI3L1.
Conclusion: Monocyte-derived alveolar macrophages are significantly expanded in SSc-ILD patients with non-end-stage fibrosis compared with HC and inversely correlate with lung function. This further supports their role in ILD pathogenesis, and posits them as potential biomarker and therapeutic target. The presence of profibrotic MoAM in HC in low numbers and their co-existence within same alveoli with non-fibrotic macrophages strongly suggest that a combination of cell-intrinsic changes and niche signaling is necessary to explain the role of MoAM in fibrosis development.
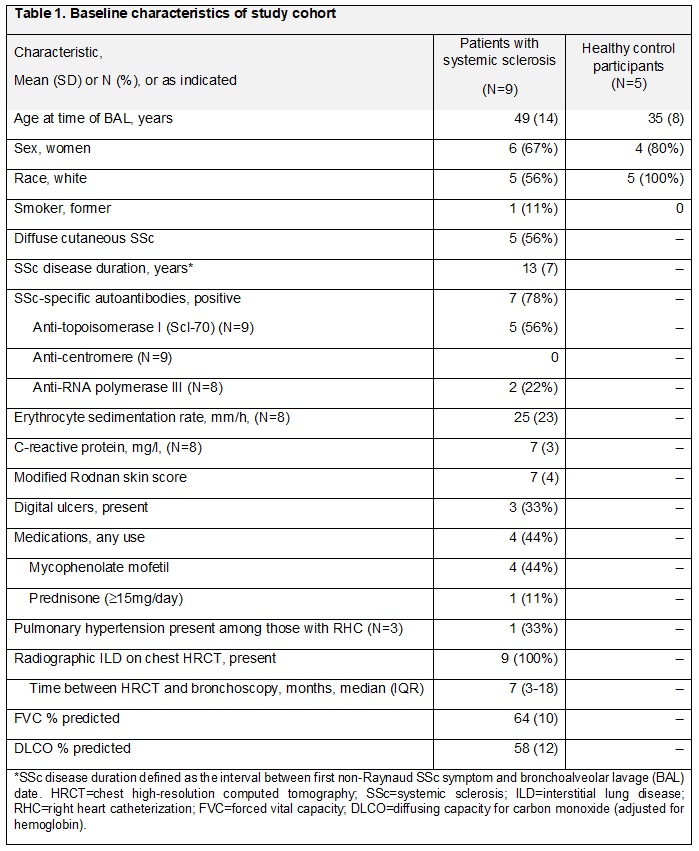
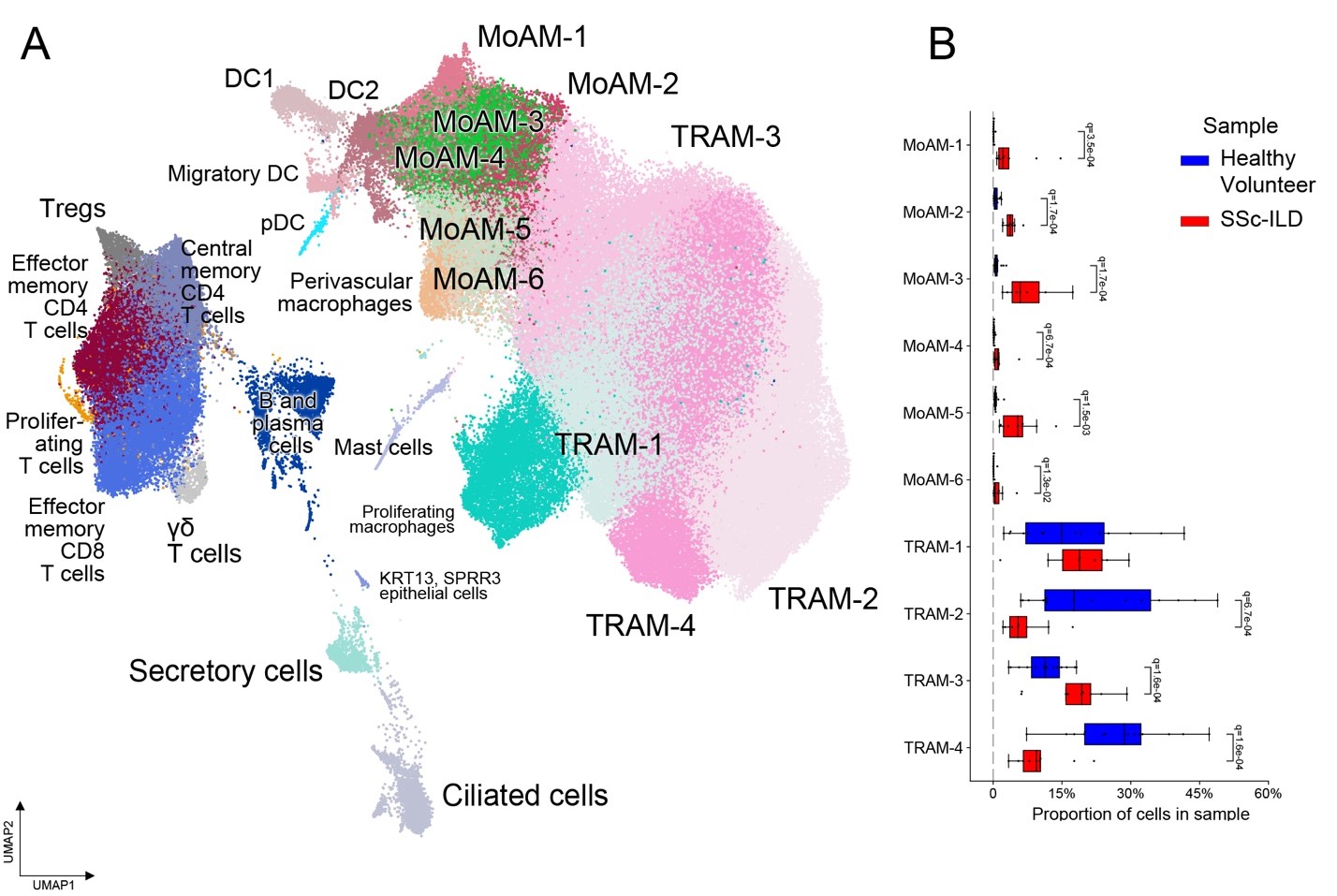 Figure 1: Monocyte-derived alveolar macrophages are expanded in SSc-ILD patients. (A) UMAP representation of cell transcriptional profiles from 25 BAL samples from 9 SSc-ILD patients, 5 healthy volunteers and 10 external published healthy volunteers, colored by cell subtype; (B) proportion of cells from TRAM and MoAM subtypes in samples.
Figure 1: Monocyte-derived alveolar macrophages are expanded in SSc-ILD patients. (A) UMAP representation of cell transcriptional profiles from 25 BAL samples from 9 SSc-ILD patients, 5 healthy volunteers and 10 external published healthy volunteers, colored by cell subtype; (B) proportion of cells from TRAM and MoAM subtypes in samples.
Disclosures: N. Markov, None; K. Senkow, None; A. Esposito, None; J. Puchalski, None; M. Gulati, France foundation, Boehringer-Ingelheim, Pulmonary fibrosis foundation, Humanetics, Bms; E. Herzog, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), sanofi, Janssen; D. Antin-Ozerkis, Boehringer Ingelheim, Fibrogen, Pliant, Galecto, Galapagos, Genentech/Roche, BMS; M. Carns, None; A. Williams, None; N. Page, None; A. Misharin, None; M. Hinchcliff, Kadmon.
Background/Purpose: Interstitial lung disease (ILD) is a leading cause of death in patients with systemic sclerosis (SSc). Previously, profibrotic monocyte-derived alveolar macrophages (MoAM) expressing (SPP1, MMP9, CHIT1, CHI3L1) have been shown to play a causal role in animal models of pulmonary fibrosis and were expanded in the lungs of SSc patients with lung fibrosis undergoing lung transplantation. We hypothesized that pro-fibrotic MoAM will be also present in SSc-ILD patients without end-stage fibrosis, and that their abundance and gene expression levels will correlate with a decline in lung function.
Methods: Nine patients with SSc and 5 healthy controls (HC) were prospectively recruited at two academic medical centers to undergo bronchoalveolar lavage (BAL). Single-cell transcriptomic (scRNA-seq) analysis of isolated immune cells was performed, and data were integrated with previously published data from 10 HC from Mould et al., 2020. Confirmatory immunohistochemistry and sm-FISH staining for macrophage subtype markers were conducted in SSc-ILD lung explants and donor lungs. Numbers of pro-fibrotic MoAM were compared to pulmonary function tests (PFT) results using Pearson correlation.
Results: Integrative analysis of scRNA-seq data of BAL samples from 9 SSc-ILD patients, 15 HC identified distinct transcriptional subsets of tissue-resident alveolar macrophages (TRAM) and MoAM. MoAM were also present in HC but were more abundant in SSc-ILD patients. Two MoAM subpopulations were characterized by expression of genes (SPP1, MMP9, CHIT1, CHI3L1) causally associated with pulmonary fibrosis. Moreover, the abundance of MoAM subpopulations in BAL from patients with SSc showed significant inverse correlation (r=−0.802±0.048) with lung function as measured by forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). Staining SSc-ILD lung explant sections for markers of profibrotic MoAM subpopulations revealed that pro-fibrotic macrophages co-exist within the same alveoli with non-fibrotic macrophages defined by lack of expression of SPP1, MMP9, CHIT1, CHI3L1.
Conclusion: Monocyte-derived alveolar macrophages are significantly expanded in SSc-ILD patients with non-end-stage fibrosis compared with HC and inversely correlate with lung function. This further supports their role in ILD pathogenesis, and posits them as potential biomarker and therapeutic target. The presence of profibrotic MoAM in HC in low numbers and their co-existence within same alveoli with non-fibrotic macrophages strongly suggest that a combination of cell-intrinsic changes and niche signaling is necessary to explain the role of MoAM in fibrosis development.

 Figure 1: Monocyte-derived alveolar macrophages are expanded in SSc-ILD patients. (A) UMAP representation of cell transcriptional profiles from 25 BAL samples from 9 SSc-ILD patients, 5 healthy volunteers and 10 external published healthy volunteers, colored by cell subtype; (B) proportion of cells from TRAM and MoAM subtypes in samples.
Figure 1: Monocyte-derived alveolar macrophages are expanded in SSc-ILD patients. (A) UMAP representation of cell transcriptional profiles from 25 BAL samples from 9 SSc-ILD patients, 5 healthy volunteers and 10 external published healthy volunteers, colored by cell subtype; (B) proportion of cells from TRAM and MoAM subtypes in samples.Disclosures: N. Markov, None; K. Senkow, None; A. Esposito, None; J. Puchalski, None; M. Gulati, France foundation, Boehringer-Ingelheim, Pulmonary fibrosis foundation, Humanetics, Bms; E. Herzog, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), sanofi, Janssen; D. Antin-Ozerkis, Boehringer Ingelheim, Fibrogen, Pliant, Galecto, Galapagos, Genentech/Roche, BMS; M. Carns, None; A. Williams, None; N. Page, None; A. Misharin, None; M. Hinchcliff, Kadmon.

