Back
Poster Session B
Session: (0752–0778) Immunological Complications of Medical Therapy Poster
0759: Immunogenicity of COVID-19 Vaccines in Patients with Autoimmune and Inflammatory Rheumatic Diseases (AIIRD) on Immunomodulatory Therapies (IMT): An Updated Cohort Study
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- GL
Gordon Lam, MD
Medical Director, Clinical Research
Arthritis and Osteoporosis Consultants of the Carolinas
Cornelius, NC, United States
Abstract Poster Presenter(s)
Gordon Lam1, Andrew Laster1, Heather Gladue1, Ahmad Kashif1, Erin Siceloff2, Victoria Lackey1, Cheryl Robertson1, Ashley Toci1, Maggie McCarter3 and Leonard Calabrese4, 1Arthritis & Osteoporosis Consultants of the Carolinas, Charlotte, NC, 2Arthritis and Osteoporosis Consultants of the Carolinas, Stallings, NC, 3University of South Carolina, Columbia, SC, 4Cleveland Clinic, Cleveland, OH
Background/Purpose: AIIRD patients may have a blunted immune response to the COVID-19 vaccines, but this is uncertain as these individuals were not included in clinical trials. To better understand the immunogenicity of the COVID-19 vaccines in this population and the effects of systemic IMT used to treat them, we quantified humoral responses to the SARS-CoV-2 spike protein in AIIRD and control patients. This is an updated report of the pilot study presented at ACRC 2021.
Methods: This prospective cohort study enrolled patients from a single-specialty practice in Charlotte NC who received the BNT162b2 (Pfizer), mRNA-1273 (Moderna), or Ad26COV2.S (Johnson & Johnson) vaccine from 3/17/21-10/1/21. Those with prior COVID-19 by self-report were excluded. We collected demographics, diagnoses, and IMT of AIIRD patients. Antibody (Ab) testing was performed at >14 days after completion of their vaccine series. SARS-CoV-2 IgG Ab levels to the S1 spike antigen RBD were measured using a semi-quantitative assay (Siemens Atellica; Quest). Associations between clinical characteristics and Ab responses were evaluated. Similar data were collected in a control group of patients with non-inflammatory disease who were not on IMT.
Results: Characteristics of 1394 AIIRD and 83 control patients are listed in Table 1. Overall, 73% of AIIRD patients developed an Ab response compared to 95% of controls (p< 0.001), with mean Ab titers of 11.59 (< 1 index) and 13.62, respectively (p< 0.001). There was no difference based on gender. The likelihood of an absent Ab response increased with age, and there was a decline in mean levels with aging in Ab+ patients. Treatment associations are listed in Table 2. Patients receiving rituximab were less likely to develop an Ab response (14%) and had a lower mean Ab titer (10.80) compared to the overall cohort. Dosing appeared to affect response rates with a single dose (1000 mg) Ab+ rate of 26% compared to a paired dose (1000 mg x2) rate of 12% (Table 3). Patients receiving abatacept were also less likely to develop an Ab response (49%). Detectable Abs were seen in most patients on JAKi (85%), belimumab (76%), TNFi (75%), IL-17i (94%), IL-6i (81%), and IL-12/23i (87%). Without concomitant targeted therapy, Ab+ rates with SSZ (69%), AZA (69%), LEF (63%), and MMF (56%) were decreased compared to MTX (82%) and HCQ (78%). 78% of patients receiving prednisone without biologics were Ab+ (average daily dose 5.8 mg). Overall dose effect is shown in Table 3.
Conclusion: AIIRD patients developed anti-SARS-CoV-2 RBD Abs at a decreased rate (73%) compared to controls (95%) with a lesser mean titer. Overall Ab response rates and titers were age dependent. Patients receiving rituximab and abatacept were less likely to develop an Ab response with the former having a dose effect. Among csDMARDs, response rates were decreased in patients receiving SSZ, AZA, LEF, and MMF. Low dose prednisone without biologics did not appear to influence Ab+ rates. Limitations of this study include its single center, non-randomized design and lack of residual confounder measures. Additional measures of humoral immune response and T cell function are also needed. However, this real-world study may provide further guidance for COVID-19 vaccination in patients with AIIRD on IMT.
.jpg) Table 1. Demographics and Clinical Characteristics of Patients with Autoimmune and Inflammatory Rheumatic Disease patients and control patients
Table 1. Demographics and Clinical Characteristics of Patients with Autoimmune and Inflammatory Rheumatic Disease patients and control patients
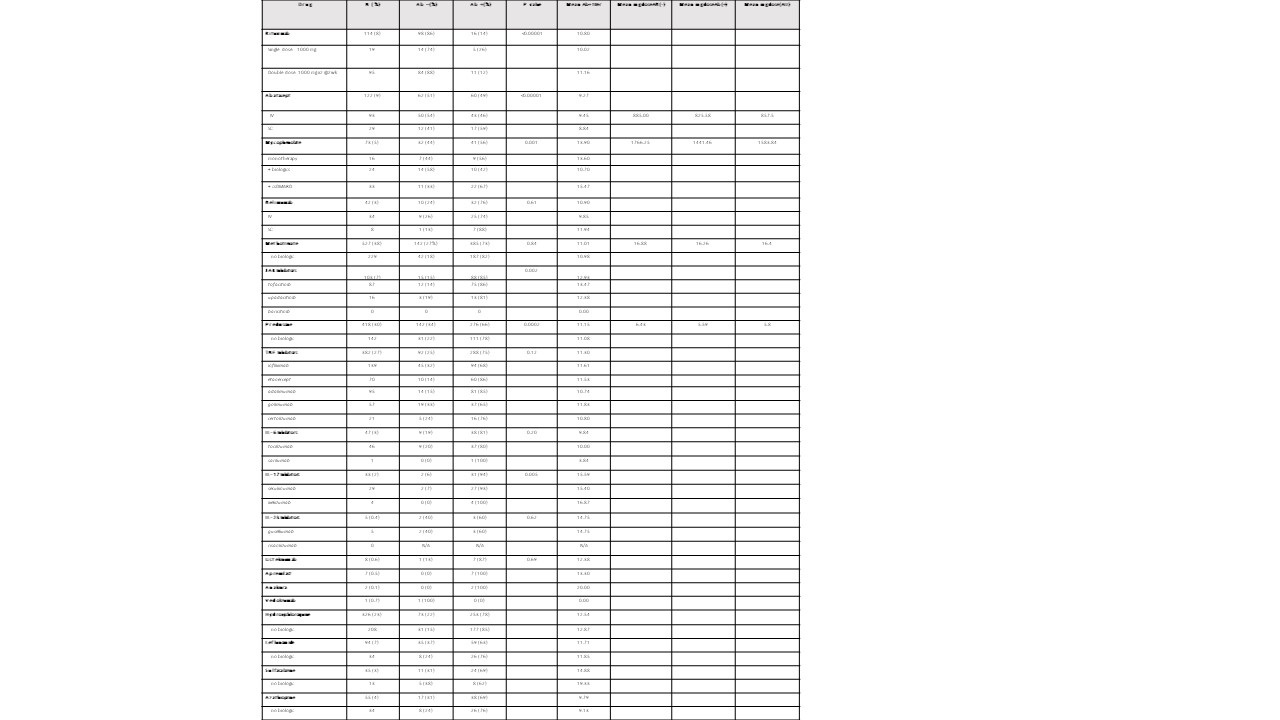 Table 2. Patient immunomodulatory therapies and vaccine responses. IgG antibodies to SARS CoV-2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov-2-igg-assay
Table 2. Patient immunomodulatory therapies and vaccine responses. IgG antibodies to SARS CoV-2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov-2-igg-assay
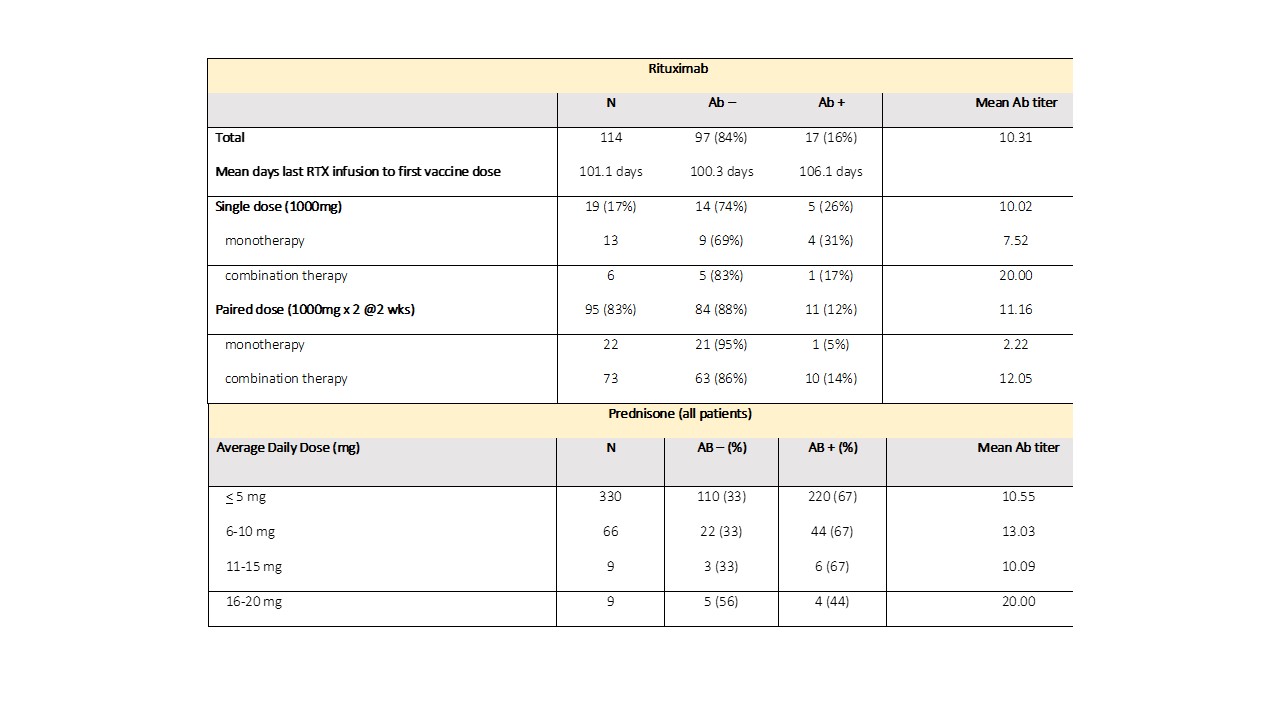 Table 3. Dose effects of rituximab and prednisone on vaccine responses in AIIRD patients
Table 3. Dose effects of rituximab and prednisone on vaccine responses in AIIRD patients
Disclosures: G. Lam, AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, UCB, AstraZeneca, Sanofi, Aurinia, Horizon; A. Laster, Amgen, Pfizer, Novartis, Eli Lilly, Chemocentryx, Exagen, Scipher, United Rheumatology; H. Gladue, GlaxoSmithKlein(GSK), AstraZeneca; A. Kashif, None; E. Siceloff, None; V. Lackey, None; C. Robertson, None; A. Toci, None; M. McCarter, None; L. Calabrese, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Genentech, Janssen, UCB, Sanofi, Regeneron, Galvani, GlaxoSmithKlein(GSK), AstraZeneca, Chemocentryx.
Background/Purpose: AIIRD patients may have a blunted immune response to the COVID-19 vaccines, but this is uncertain as these individuals were not included in clinical trials. To better understand the immunogenicity of the COVID-19 vaccines in this population and the effects of systemic IMT used to treat them, we quantified humoral responses to the SARS-CoV-2 spike protein in AIIRD and control patients. This is an updated report of the pilot study presented at ACRC 2021.
Methods: This prospective cohort study enrolled patients from a single-specialty practice in Charlotte NC who received the BNT162b2 (Pfizer), mRNA-1273 (Moderna), or Ad26COV2.S (Johnson & Johnson) vaccine from 3/17/21-10/1/21. Those with prior COVID-19 by self-report were excluded. We collected demographics, diagnoses, and IMT of AIIRD patients. Antibody (Ab) testing was performed at >14 days after completion of their vaccine series. SARS-CoV-2 IgG Ab levels to the S1 spike antigen RBD were measured using a semi-quantitative assay (Siemens Atellica; Quest). Associations between clinical characteristics and Ab responses were evaluated. Similar data were collected in a control group of patients with non-inflammatory disease who were not on IMT.
Results: Characteristics of 1394 AIIRD and 83 control patients are listed in Table 1. Overall, 73% of AIIRD patients developed an Ab response compared to 95% of controls (p< 0.001), with mean Ab titers of 11.59 (< 1 index) and 13.62, respectively (p< 0.001). There was no difference based on gender. The likelihood of an absent Ab response increased with age, and there was a decline in mean levels with aging in Ab+ patients. Treatment associations are listed in Table 2. Patients receiving rituximab were less likely to develop an Ab response (14%) and had a lower mean Ab titer (10.80) compared to the overall cohort. Dosing appeared to affect response rates with a single dose (1000 mg) Ab+ rate of 26% compared to a paired dose (1000 mg x2) rate of 12% (Table 3). Patients receiving abatacept were also less likely to develop an Ab response (49%). Detectable Abs were seen in most patients on JAKi (85%), belimumab (76%), TNFi (75%), IL-17i (94%), IL-6i (81%), and IL-12/23i (87%). Without concomitant targeted therapy, Ab+ rates with SSZ (69%), AZA (69%), LEF (63%), and MMF (56%) were decreased compared to MTX (82%) and HCQ (78%). 78% of patients receiving prednisone without biologics were Ab+ (average daily dose 5.8 mg). Overall dose effect is shown in Table 3.
Conclusion: AIIRD patients developed anti-SARS-CoV-2 RBD Abs at a decreased rate (73%) compared to controls (95%) with a lesser mean titer. Overall Ab response rates and titers were age dependent. Patients receiving rituximab and abatacept were less likely to develop an Ab response with the former having a dose effect. Among csDMARDs, response rates were decreased in patients receiving SSZ, AZA, LEF, and MMF. Low dose prednisone without biologics did not appear to influence Ab+ rates. Limitations of this study include its single center, non-randomized design and lack of residual confounder measures. Additional measures of humoral immune response and T cell function are also needed. However, this real-world study may provide further guidance for COVID-19 vaccination in patients with AIIRD on IMT.
.jpg) Table 1. Demographics and Clinical Characteristics of Patients with Autoimmune and Inflammatory Rheumatic Disease patients and control patients
Table 1. Demographics and Clinical Characteristics of Patients with Autoimmune and Inflammatory Rheumatic Disease patients and control patients  Table 2. Patient immunomodulatory therapies and vaccine responses. IgG antibodies to SARS CoV-2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov-2-igg-assay
Table 2. Patient immunomodulatory therapies and vaccine responses. IgG antibodies to SARS CoV-2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov-2-igg-assay Table 3. Dose effects of rituximab and prednisone on vaccine responses in AIIRD patients
Table 3. Dose effects of rituximab and prednisone on vaccine responses in AIIRD patientsDisclosures: G. Lam, AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, UCB, AstraZeneca, Sanofi, Aurinia, Horizon; A. Laster, Amgen, Pfizer, Novartis, Eli Lilly, Chemocentryx, Exagen, Scipher, United Rheumatology; H. Gladue, GlaxoSmithKlein(GSK), AstraZeneca; A. Kashif, None; E. Siceloff, None; V. Lackey, None; C. Robertson, None; A. Toci, None; M. McCarter, None; L. Calabrese, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Genentech, Janssen, UCB, Sanofi, Regeneron, Galvani, GlaxoSmithKlein(GSK), AstraZeneca, Chemocentryx.

