Back
Abstract Session
Session: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases II (1106–1111)
1109: High Interleukin-13 Level Is Associated with Disease Stability in Interstitial Lung Disease
Sunday, November 13, 2022
11:15 AM – 11:25 AM Eastern Time
Location: Room 119
- EJ
Elena Joerns, MD
UT Southwestern
Dallas, TX, United States
Presenting Author(s)
Elena Joerns1, David Karp1, Jeffrey Sparks2, Traci Adams1, Una Makris3 and Chad Newton1, 1UT Southwestern, Dallas, TX, 2Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 3UT Southwestern Medical Center and Dallas VA, Dallas, TX
Background/Purpose: Interstitial lung disease (ILD) is a disease of lung parenchyma manifesting with inflammation and fibrosis. Interstitial lung disease (ILD) is a frequent complication of rheumatic diseases (RDs) but other types exist. Biomarkers can help differentiate between ILD subtype and predict prognosis. The objectives of our study were to 1) compare baseline cytokine levels between ILD subtypes, 2) evaluate baseline differences in cytokines between ILD patients exhibiting progressive phenotype and those without progression, and 3) evaluate differences between progressors and non-progressors within each ILD subtype.
Methods: We quantified 27 cytokines using Multiplex assay in peripheral blood samples from 234 patients with ILD (chronic hypersensitivity pneumonitis [cHP], RD-ILD, idiopathic pneumonia with autoimmune features [IPAF], idiopathic pulmonary fibrosis [IPF], and unclassifiable ILD [uILD]). Patients who were not on treatment (n=74) during sample collection were evaluated separately. Pairwise comparisons using Tukey method were performed to assess significant differences in cytokine levels between ILD subtypes. Multivariable logistic regression was performed to evaluate cytokine impact on the odds of progression. Student's t-test was employed to evaluate differences in cytokine levels between progressors and non-progressors within each ILD subtype. As an exploratory analysis, multivariable logistic regression was utilized to evaluate cytokines predicting having an inflammatory ILD type (cHP and RD-ILD) as opposed to predominantly fibrotic (IPF) type.
Results: We found that baseline IL-17 level differed significantly between RD-ILD, IPF, and IPAF, being highest in RD-ILD and lowest in IPAF. Additionally, higher IL-13 level was strongly associated with increased odds of non-progression in our cohort (odds ratio 0.41, 95% confidence interval 0.20-0.85), with the levels being similar in non-progressors in all ILD types evaluated. FGF-b, GM-CSF, IL-12, and IL-17 were associated with greater odds of having inflammatory ILD type, rather than predominantly fibrotic type, even after adjusting for fibrosis severity and usual interstitial pneumonia radiographic pattern.
Conclusion: Investigations of biomarker differences between ILD subtypes is important, as accurate classification of ILD has clinical implications for treatment and management. IL-13 may be a useful biomarker predicting ILD stability. Further research is warranted to investigate cytokine levels within progressive and non-progressive ILD phenotypes with the goal of identifying biomarkers predicting progression and elucidating underlying pathogenesis. Future larger scale, prospective studies may help address both of these goals.
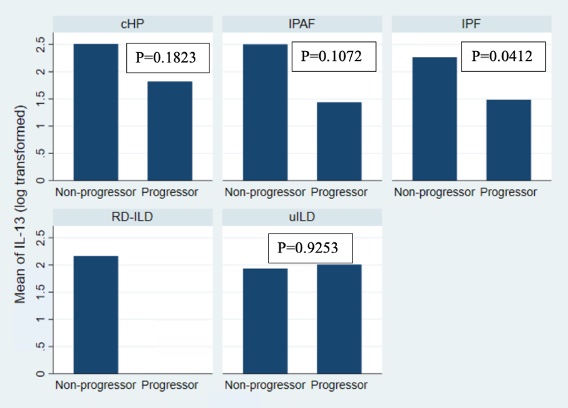 Figure 1: Mean IL-13 level comparison by progressor status for each ILD subtype, untreated patient cohort.
Figure 1: Mean IL-13 level comparison by progressor status for each ILD subtype, untreated patient cohort.
IL-13 – interleukin 13, ILD – interstitial lung disease, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD.
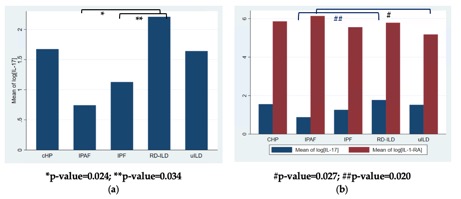 Figure 2. Cytokine level differences between ILD subtypes; (a) IL-17 level differs significantly between IPAF and RD-ILD and between RD-ILD and IPF in untreated cohort; (b) IL-17 differs significantly between IPAF and RD-ILD and IL-1-RA level differs significantly between IPAF and uILD in total cohort. [Log - natural log, IL – interleukin, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD].
Figure 2. Cytokine level differences between ILD subtypes; (a) IL-17 level differs significantly between IPAF and RD-ILD and between RD-ILD and IPF in untreated cohort; (b) IL-17 differs significantly between IPAF and RD-ILD and IL-1-RA level differs significantly between IPAF and uILD in total cohort. [Log - natural log, IL – interleukin, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD].
Disclosures: E. Joerns, Pfizer, Inc; D. Karp, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; T. Adams, None; U. Makris, None; C. Newton, Boehringer-Ingelheim.
Background/Purpose: Interstitial lung disease (ILD) is a disease of lung parenchyma manifesting with inflammation and fibrosis. Interstitial lung disease (ILD) is a frequent complication of rheumatic diseases (RDs) but other types exist. Biomarkers can help differentiate between ILD subtype and predict prognosis. The objectives of our study were to 1) compare baseline cytokine levels between ILD subtypes, 2) evaluate baseline differences in cytokines between ILD patients exhibiting progressive phenotype and those without progression, and 3) evaluate differences between progressors and non-progressors within each ILD subtype.
Methods: We quantified 27 cytokines using Multiplex assay in peripheral blood samples from 234 patients with ILD (chronic hypersensitivity pneumonitis [cHP], RD-ILD, idiopathic pneumonia with autoimmune features [IPAF], idiopathic pulmonary fibrosis [IPF], and unclassifiable ILD [uILD]). Patients who were not on treatment (n=74) during sample collection were evaluated separately. Pairwise comparisons using Tukey method were performed to assess significant differences in cytokine levels between ILD subtypes. Multivariable logistic regression was performed to evaluate cytokine impact on the odds of progression. Student's t-test was employed to evaluate differences in cytokine levels between progressors and non-progressors within each ILD subtype. As an exploratory analysis, multivariable logistic regression was utilized to evaluate cytokines predicting having an inflammatory ILD type (cHP and RD-ILD) as opposed to predominantly fibrotic (IPF) type.
Results: We found that baseline IL-17 level differed significantly between RD-ILD, IPF, and IPAF, being highest in RD-ILD and lowest in IPAF. Additionally, higher IL-13 level was strongly associated with increased odds of non-progression in our cohort (odds ratio 0.41, 95% confidence interval 0.20-0.85), with the levels being similar in non-progressors in all ILD types evaluated. FGF-b, GM-CSF, IL-12, and IL-17 were associated with greater odds of having inflammatory ILD type, rather than predominantly fibrotic type, even after adjusting for fibrosis severity and usual interstitial pneumonia radiographic pattern.
Conclusion: Investigations of biomarker differences between ILD subtypes is important, as accurate classification of ILD has clinical implications for treatment and management. IL-13 may be a useful biomarker predicting ILD stability. Further research is warranted to investigate cytokine levels within progressive and non-progressive ILD phenotypes with the goal of identifying biomarkers predicting progression and elucidating underlying pathogenesis. Future larger scale, prospective studies may help address both of these goals.
 Figure 1: Mean IL-13 level comparison by progressor status for each ILD subtype, untreated patient cohort.
Figure 1: Mean IL-13 level comparison by progressor status for each ILD subtype, untreated patient cohort.IL-13 – interleukin 13, ILD – interstitial lung disease, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD.
 Figure 2. Cytokine level differences between ILD subtypes; (a) IL-17 level differs significantly between IPAF and RD-ILD and between RD-ILD and IPF in untreated cohort; (b) IL-17 differs significantly between IPAF and RD-ILD and IL-1-RA level differs significantly between IPAF and uILD in total cohort. [Log - natural log, IL – interleukin, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD].
Figure 2. Cytokine level differences between ILD subtypes; (a) IL-17 level differs significantly between IPAF and RD-ILD and between RD-ILD and IPF in untreated cohort; (b) IL-17 differs significantly between IPAF and RD-ILD and IL-1-RA level differs significantly between IPAF and uILD in total cohort. [Log - natural log, IL – interleukin, cHP – chronic hypersensitivity pneumonitis, IPAF – interstitial pneumonia with autoimmune features, IPF – idiopathic pulmonary fibrosis, RD-ILD – rheumatic disease associated ILD, uILD – unclassifiable ILD].Disclosures: E. Joerns, Pfizer, Inc; D. Karp, None; J. Sparks, Bristol Myers Squibb, AbbVie/Abbott, Amgen, Boehringer Ingelheim, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer; T. Adams, None; U. Makris, None; C. Newton, Boehringer-Ingelheim.

