Back
Poster Session A
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (0150–0180) Muscle Biology, Myositis and Myopathies Poster I
0159: B Cell Phenotype in Adult Patients with Idiopathic Inflammatory Myositis (IIM) Before and After Belimumab
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GM
Galina Marder, MD
Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell
Great Neck, NY, United States
Abstract Poster Presenter(s)
Tam Quach1, Anne Davidson2 and Galina Marder3, 1Feinstein Institute for Medical Research, Manhasset, NY, 2Feinstein Institutes for Medical Research, Manhasset, NY, 3Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Great Neck, NY
Background/Purpose: Recent observations suggest the importance of the BAFF pathway in the pathogenesis of Idiopathic Inflammatory Myositis (IIM). Elevated serum levels of circulating BAFF in patients with myositis independently correlate with myositis disease activity. Muscle tissues of IIM patients have marked up-regulation of the BAFF transcript, and local B and plasma cells have high expression of BAFF receptors (BAFFR). This study aimed to evaluate the B cell phenotype in IIM patients before and after treatment with belimumab.
Methods: Samples were available from 10 patients that participated in a 40-week multicenter randomized, double blind, placebo-controlled trial of belimumab with a 24-week open label extension for adults with refractory IIM, meeting ACR 2017 classification criteria of polymyositis/ dermatomyositis (PM/DM). Patients were randomized 1:1 to IV belimumab 10mg/kg or placebo for 40 weeks followed by 24 weeks open label phase with belimumab 10mg/kg. The clinical response was measured as change in total improvement score (TIS) before starting belimumab and after. For the mechanistic portion of the study the baseline sample (D0) of each patient served as a paired control for samples obtained 6 months and 15 months after receiving belimumab. The number and % of B cells and B cell subsets (transitional, naïve, double negative, memory, and plasmablasts) were analyzed using flow cytometry.
Descriptive statistics, the Mann-Whitney test, Fisher's exact, and ANOVA Friedman tests were used to compare groups for continuous and categorical variables, respectively.
Results: Demographic, clinical characteristics, and response to belimumab treatment of 10 patients with available samples for B cell analyses are shown in Table 1. 30% were on belimumab for 6mo and had no clinical response (TIS = -15-4.5). Clinical responses ranged from minimal to major (TIS = 27.5- 72.5) in 6/7 patients that had 10mo of belimumab. Only 2 patients had major improvement (TIS -72.5). Samples from 10 subjects were available for phenotype analysis by flow cytometry. At baseline, the % of B cells in the live lymphocyte population was 11.3 (± 7.8), comparable to healthy donors. After treatment there was no reduction in the absolute count of B cells at 6mo compared pre-treatment levels and only a trend towards reduction at 15mo (p< 0.06). There was a shift in the distribution of B cell subsets with a significant reduction in transitional T3 and naïve B cell compartments and a significant increase in unswitched and switched memory B cells. There was no significant change in the % or number of IgMlo (anergic) naïve B cells, ANA+ B cells, plasmablasts, CD3 T cells and non-B, non-T cells after treatment.
Conclusion: The baseline B cell phenotype in IIM patients was similar to that reported in healthy donors. No clinical or biological effect was seen at 4mo on belimumab. Absolute B cell counts only modestly decreased even after 15mo of belimumab. As reported for other diseases, belimumab induced depletion in the transitional T3 and naïve B cell compartments while enhancing the memory B cell compartments.
Supported by GSK grant and NIH1R21AR070540-01
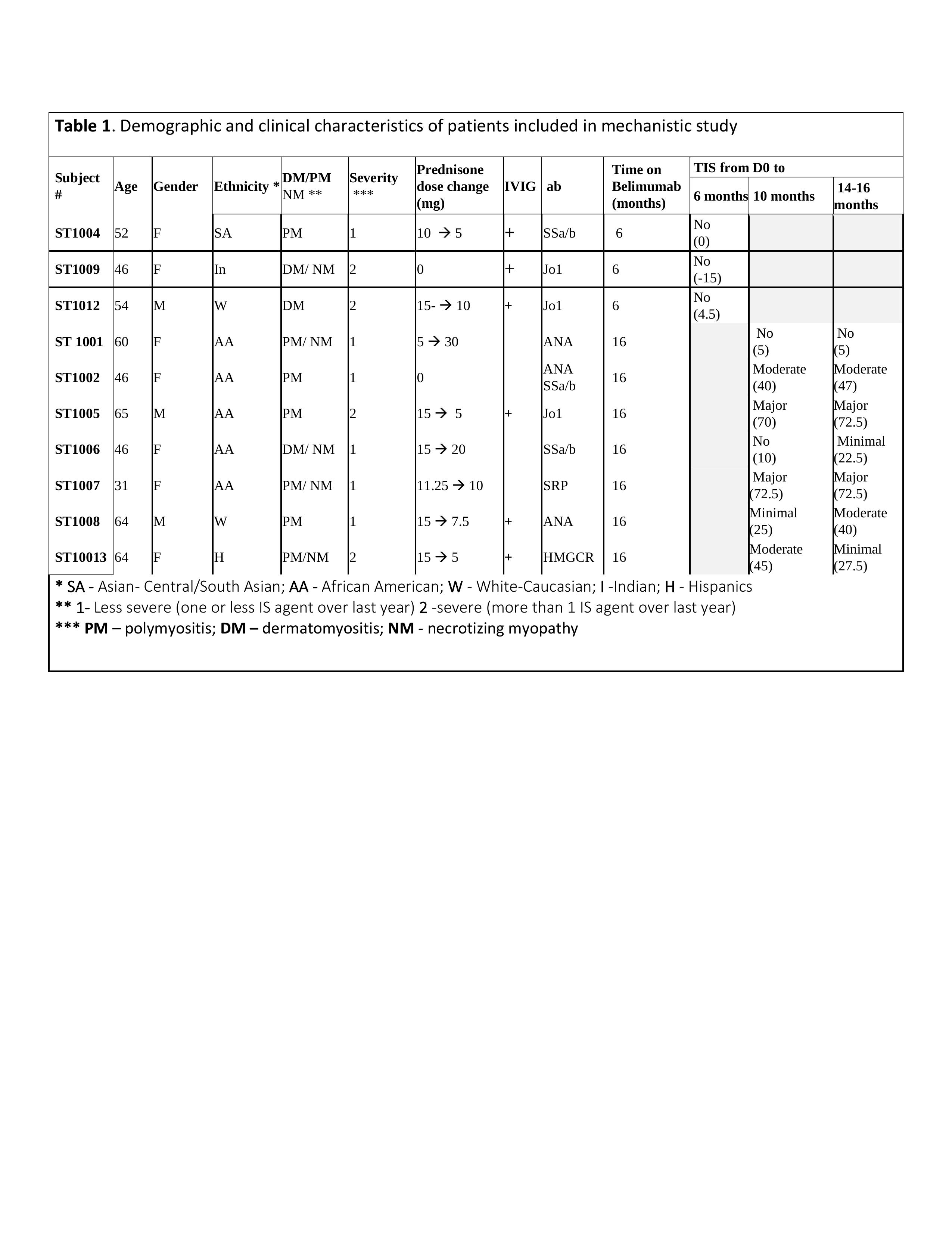
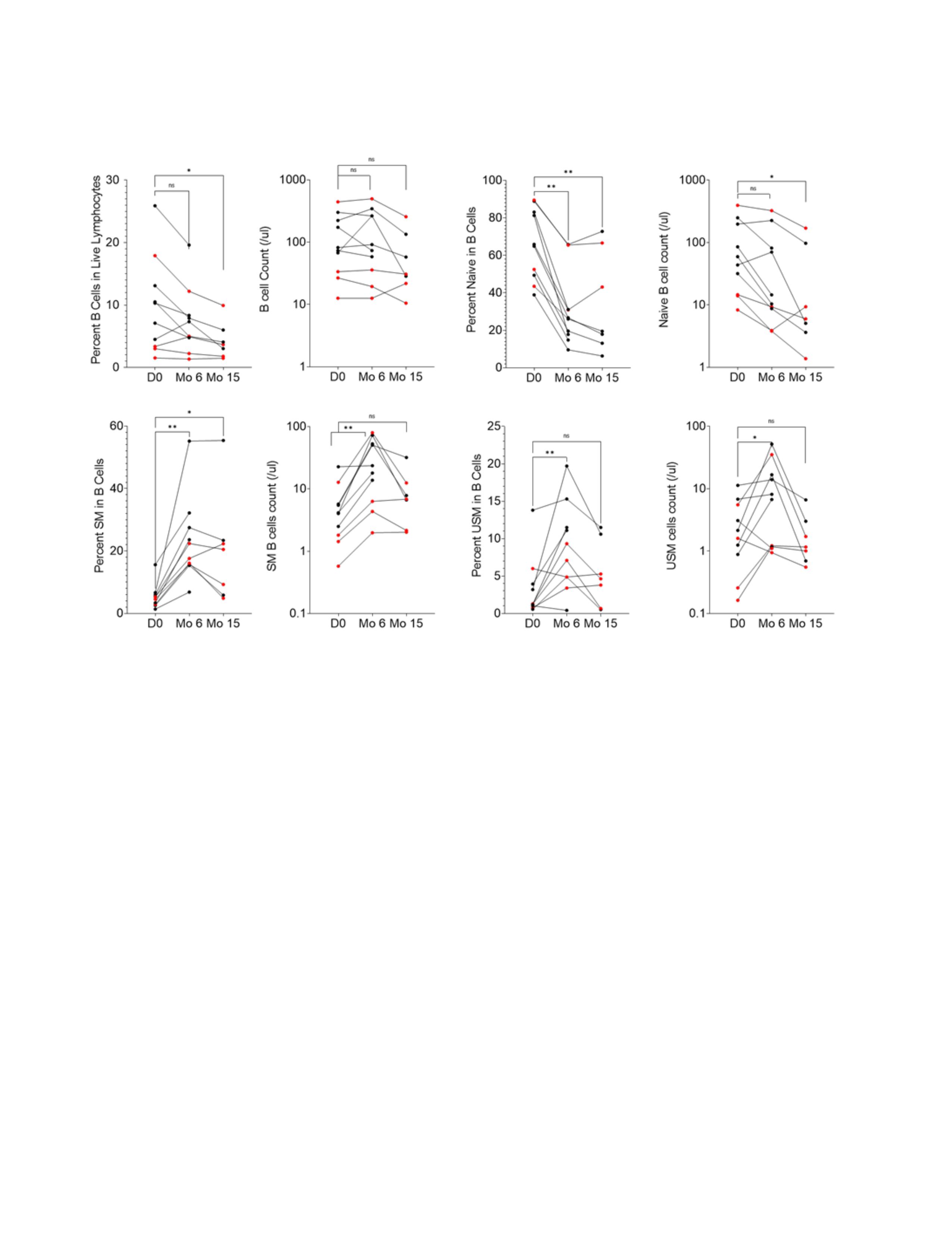 Figure 1. A-C. Plots show percent and number of B cells (A), Naive (B) Switched Memory (SM) (C) and unswitched memory (USM) (D) B cells in peripheral blood of IIM patients before (D0), 6 months (Mo) and 15 months after Belimumab treatment (n=7). Shown in red are patients who reached moderate or major TIS after the treatment. ANOVA Friedman test with Dunn’s multiple comparisons, *p < 0.05, **p < 0.005, ns = p not significant.
Figure 1. A-C. Plots show percent and number of B cells (A), Naive (B) Switched Memory (SM) (C) and unswitched memory (USM) (D) B cells in peripheral blood of IIM patients before (D0), 6 months (Mo) and 15 months after Belimumab treatment (n=7). Shown in red are patients who reached moderate or major TIS after the treatment. ANOVA Friedman test with Dunn’s multiple comparisons, *p < 0.05, **p < 0.005, ns = p not significant.
Disclosures: T. Quach, None; A. Davidson, None; G. Marder, GlaxoSmithKlein(GSK).
Background/Purpose: Recent observations suggest the importance of the BAFF pathway in the pathogenesis of Idiopathic Inflammatory Myositis (IIM). Elevated serum levels of circulating BAFF in patients with myositis independently correlate with myositis disease activity. Muscle tissues of IIM patients have marked up-regulation of the BAFF transcript, and local B and plasma cells have high expression of BAFF receptors (BAFFR). This study aimed to evaluate the B cell phenotype in IIM patients before and after treatment with belimumab.
Methods: Samples were available from 10 patients that participated in a 40-week multicenter randomized, double blind, placebo-controlled trial of belimumab with a 24-week open label extension for adults with refractory IIM, meeting ACR 2017 classification criteria of polymyositis/ dermatomyositis (PM/DM). Patients were randomized 1:1 to IV belimumab 10mg/kg or placebo for 40 weeks followed by 24 weeks open label phase with belimumab 10mg/kg. The clinical response was measured as change in total improvement score (TIS) before starting belimumab and after. For the mechanistic portion of the study the baseline sample (D0) of each patient served as a paired control for samples obtained 6 months and 15 months after receiving belimumab. The number and % of B cells and B cell subsets (transitional, naïve, double negative, memory, and plasmablasts) were analyzed using flow cytometry.
Descriptive statistics, the Mann-Whitney test, Fisher's exact, and ANOVA Friedman tests were used to compare groups for continuous and categorical variables, respectively.
Results: Demographic, clinical characteristics, and response to belimumab treatment of 10 patients with available samples for B cell analyses are shown in Table 1. 30% were on belimumab for 6mo and had no clinical response (TIS = -15-4.5). Clinical responses ranged from minimal to major (TIS = 27.5- 72.5) in 6/7 patients that had 10mo of belimumab. Only 2 patients had major improvement (TIS -72.5). Samples from 10 subjects were available for phenotype analysis by flow cytometry. At baseline, the % of B cells in the live lymphocyte population was 11.3 (± 7.8), comparable to healthy donors. After treatment there was no reduction in the absolute count of B cells at 6mo compared pre-treatment levels and only a trend towards reduction at 15mo (p< 0.06). There was a shift in the distribution of B cell subsets with a significant reduction in transitional T3 and naïve B cell compartments and a significant increase in unswitched and switched memory B cells. There was no significant change in the % or number of IgMlo (anergic) naïve B cells, ANA+ B cells, plasmablasts, CD3 T cells and non-B, non-T cells after treatment.
Conclusion: The baseline B cell phenotype in IIM patients was similar to that reported in healthy donors. No clinical or biological effect was seen at 4mo on belimumab. Absolute B cell counts only modestly decreased even after 15mo of belimumab. As reported for other diseases, belimumab induced depletion in the transitional T3 and naïve B cell compartments while enhancing the memory B cell compartments.
Supported by GSK grant and NIH1R21AR070540-01

 Figure 1. A-C. Plots show percent and number of B cells (A), Naive (B) Switched Memory (SM) (C) and unswitched memory (USM) (D) B cells in peripheral blood of IIM patients before (D0), 6 months (Mo) and 15 months after Belimumab treatment (n=7). Shown in red are patients who reached moderate or major TIS after the treatment. ANOVA Friedman test with Dunn’s multiple comparisons, *p < 0.05, **p < 0.005, ns = p not significant.
Figure 1. A-C. Plots show percent and number of B cells (A), Naive (B) Switched Memory (SM) (C) and unswitched memory (USM) (D) B cells in peripheral blood of IIM patients before (D0), 6 months (Mo) and 15 months after Belimumab treatment (n=7). Shown in red are patients who reached moderate or major TIS after the treatment. ANOVA Friedman test with Dunn’s multiple comparisons, *p < 0.05, **p < 0.005, ns = p not significant. Disclosures: T. Quach, None; A. Davidson, None; G. Marder, GlaxoSmithKlein(GSK).

