Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0249: Identification of Distinct Peripheral Blood Myeloid Cell Subpopulations in Patients with Rheumatoid Arthritis-associated Interstitial Lung Disease
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
- JP
Jill Poole, MD
University of Nebraska Medical Center
Omaha, NE, United States
Abstract Poster Presenter(s)
Jill Poole1, Bryant England1, Kathryn Cole1, James Talmadge1, Amy Nelson1, Rohit Gaurav1, Aaron Schwabb1, Angela Gleason1, Michael Duryee1, Rhonda Walenz1, Bridget Kramer1, Joel VanDeGraaff1, Sara May1, Geoffrey Thiele1 and Ted Mikuls2, 1University of Nebraska Medical Center, Omaha, NE, 2Division of Rheumatology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: Interstitial lung disease (ILD) is associated with significant morbidity and mortality in rheumatoid arthritis (RA). The key cellular players of RA-ILD remain largely unknown. Alterations in monocyte subpopulations marked by a shift from classical to intermediate and nonclassical monocytes are associated with RA. There is little information regarding monocytic-myeloid derived suppressor cells (mMDSCs) and polymorphonuclear (PMN)-MDSCs in RA, and the studies that have been reported are conflicting. Additionally, there is little to no information on these inflammatory and/or suppressive cell populations in patients with RA-ILD. Thus, the purpose of this on-going study is to characterize peripheral blood myeloid cell subpopulations in RA and RA-ILD.
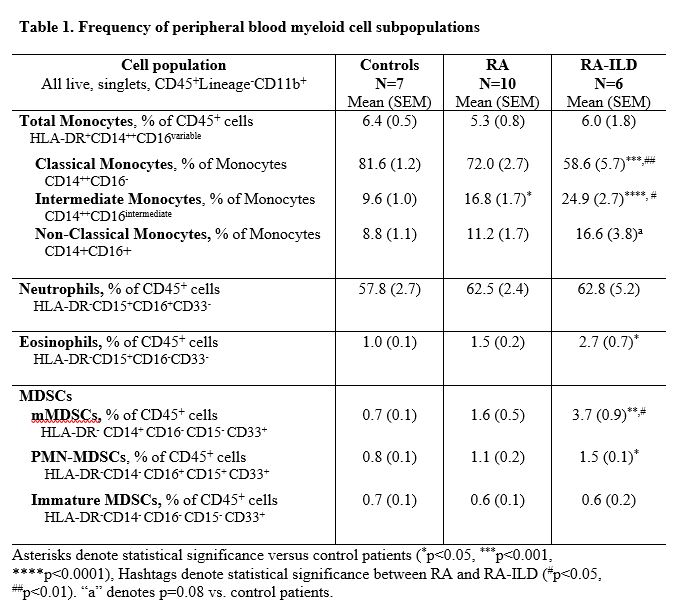
Methods: Peripheral blood collected from patients with RA (N=10, all RA fulfilled ACR classification criteria), RA-ILD (N=6, all ILD based upon clinical diagnosis and CT findings), and a convenience sample of comparator patients (N=7, without autoimmune or systemic inflammatory disease) was processed and immunophenotyped for myeloid cell subpopulations by flow cytometry on the same day as collection. After removal of debris and doublets, all myeloid cell populations were gated on live, CD45+, lineage (CD3, CD19, CD56) negative, and CD11b+ cells. Monocytes, PMNs, eosinophils, and MDSCs were delineated by expression of HLA-DR, CD14, CD16, CD15, CD33 (see Table 1). Subpopulation frequency is reported as a percentage of total CD45+ cells or of parent population (monocytes) and significant differences across groups were determined by one-way ANOVA and Tukey's post-test.
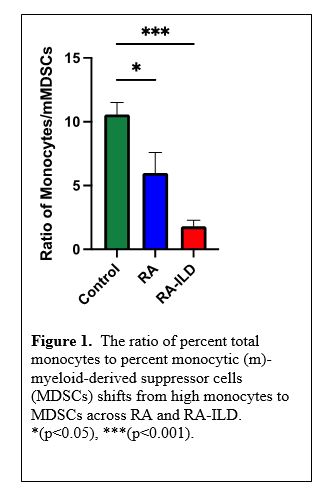
Results: The frequency and differences of the myeloid cell subpopulations across groups are shown in Table 1. Although total peripheral blood monocyte frequency did not differ across groups, we observed a significantly higher frequency of intermediate monocytes in RA and RA-ILD patients vs. control with the highest frequency observed in RA-ILD. In contrast, classical monocytes were significantly lower in patients with RA-ILD vs. both RA and control groups. Although non-significant, increased trends were observed for non-classical monocytes in RA-ILD patients. Eosinophils were significantly higher in RA-ILD vs. controls. Although there was no difference in PMNs across groups, PMN-MDSCs were significantly increased in RA-ILD vs. control groups. The frequency of mMDSCs was significantly higher in RA-ILD vs. both control and RA. Furthermore, the ratio of %monocytes to %mMDSCs differed across groups with a striking shift towards higher mMDSCs vs. monocytes in RA-ILD (Figure 1).
Conclusion: Flow cytometry-based myeloid cell immunophenotyping in patients with RA-ILD may provide comprehensive information on immune cell networks that could provide foundation for a better understanding of disease pathogenesis and more effective management. Our early preliminary results of this ongoing study suggest significant shifts in monocytic cell subpopulations with increases in intermediate/non-classical monocytes and monocytic-MDSCs as well as increases in PMN-MDSCs and eosinophils in RA-ILD, shifts that are distinct from RA alone.
Disclosures: J. Poole, AstraZeneca; B. England, Boehringer-Ingelheim; K. Cole, None; J. Talmadge, None; A. Nelson, None; R. Gaurav, None; A. Schwabb, None; A. Gleason, None; M. Duryee, None; R. Walenz, None; B. Kramer, None; J. VanDeGraaff, None; S. May, None; G. Thiele, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.
Background/Purpose: Interstitial lung disease (ILD) is associated with significant morbidity and mortality in rheumatoid arthritis (RA). The key cellular players of RA-ILD remain largely unknown. Alterations in monocyte subpopulations marked by a shift from classical to intermediate and nonclassical monocytes are associated with RA. There is little information regarding monocytic-myeloid derived suppressor cells (mMDSCs) and polymorphonuclear (PMN)-MDSCs in RA, and the studies that have been reported are conflicting. Additionally, there is little to no information on these inflammatory and/or suppressive cell populations in patients with RA-ILD. Thus, the purpose of this on-going study is to characterize peripheral blood myeloid cell subpopulations in RA and RA-ILD.
Methods: Peripheral blood collected from patients with RA (N=10, all RA fulfilled ACR classification criteria), RA-ILD (N=6, all ILD based upon clinical diagnosis and CT findings), and a convenience sample of comparator patients (N=7, without autoimmune or systemic inflammatory disease) was processed and immunophenotyped for myeloid cell subpopulations by flow cytometry on the same day as collection. After removal of debris and doublets, all myeloid cell populations were gated on live, CD45+, lineage (CD3, CD19, CD56) negative, and CD11b+ cells. Monocytes, PMNs, eosinophils, and MDSCs were delineated by expression of HLA-DR, CD14, CD16, CD15, CD33 (see Table 1). Subpopulation frequency is reported as a percentage of total CD45+ cells or of parent population (monocytes) and significant differences across groups were determined by one-way ANOVA and Tukey's post-test.
Results: The frequency and differences of the myeloid cell subpopulations across groups are shown in Table 1. Although total peripheral blood monocyte frequency did not differ across groups, we observed a significantly higher frequency of intermediate monocytes in RA and RA-ILD patients vs. control with the highest frequency observed in RA-ILD. In contrast, classical monocytes were significantly lower in patients with RA-ILD vs. both RA and control groups. Although non-significant, increased trends were observed for non-classical monocytes in RA-ILD patients. Eosinophils were significantly higher in RA-ILD vs. controls. Although there was no difference in PMNs across groups, PMN-MDSCs were significantly increased in RA-ILD vs. control groups. The frequency of mMDSCs was significantly higher in RA-ILD vs. both control and RA. Furthermore, the ratio of %monocytes to %mMDSCs differed across groups with a striking shift towards higher mMDSCs vs. monocytes in RA-ILD (Figure 1).
Conclusion: Flow cytometry-based myeloid cell immunophenotyping in patients with RA-ILD may provide comprehensive information on immune cell networks that could provide foundation for a better understanding of disease pathogenesis and more effective management. Our early preliminary results of this ongoing study suggest significant shifts in monocytic cell subpopulations with increases in intermediate/non-classical monocytes and monocytic-MDSCs as well as increases in PMN-MDSCs and eosinophils in RA-ILD, shifts that are distinct from RA alone.


Disclosures: J. Poole, AstraZeneca; B. England, Boehringer-Ingelheim; K. Cole, None; J. Talmadge, None; A. Nelson, None; R. Gaurav, None; A. Schwabb, None; A. Gleason, None; M. Duryee, None; R. Walenz, None; B. Kramer, None; J. VanDeGraaff, None; S. May, None; G. Thiele, None; T. Mikuls, Gilead Sciences, Bristol-Myers Squibb, Horizon, Sanofi, Pfizer Inc.

