Back
Clinical Research
(CR-012) Updated Results from an Open Phase 2 Study Assessing the Safety, Efficacy and Pharmacological Effects of Bromelain-based Enzymatic Debridement on Biofilm, Microbial loads and Cytokines in patients with DFU and VLU

Co-Author(s):
Robert Snyder, DPM, MBA, MSc, CWSP
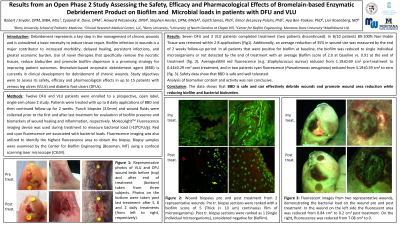
<b>Introduction</b>: <p class="Default"><span style="font-size: 11.0pt;">Debridement represents a key step in the management of chronic wounds and is considered a basic necessity to induce tissue repair. Biofilm infection in wounds is a major contributor to increased morbidity, delayed healing, persistent infections and greater economic burden. Use of novel therapies that specifically remove the necrotic tissues, reduce bioburden and promote biofilm dispersion is a promising strategy for improving patient outcomes. Bromelain-based enzymatic debridement agent (BBD) is currently in clinical development for debridement of chronic wounds. Study objectives are to assess its safety, efficacy and pharmacological effects in patients with venous leg ulcers and diabetic foot ulcers.<span style="mso-spacerun: yes;"> </span></span></p><br/><br/><b>Methods</b>: <p class="MsoNormal" style="margin-top: 6.0pt;"><span style="font-family: 'Times New Roman',serif; color: black;">12 DFU and VLU patients were enrolled to a prospective, open-label, single-arm phase-2 study. Patients were treated with up to 8 daily applications of BBD and then continued follow-up for 2-weeks.</span> <span style="font-family: 'Times New Roman',serif; color: black;">Punch biopsies (3.0mm) and wound fluids were collected prior to the first and after last treatment. Biofilm presence was analyzed from wound biopsies. Wound fluid was analyzed for evaluation of biomarkers of wound healing and inflammation, i.e. MMPs, cytokines, growth-factors, etc. Fluorescence imaging device was used during treatment to measure bacterial load ( >10<sup>4</sup>CFU/g). Fluorescence imaging was also utilized to identify the highest fluorescence area to obtain the biopsy.</span></p><br/><br/><b>Results</b>: <p class="MsoNoSpacing" style="text-align: justify;"><span style="font-family: 'Times New Roman',serif;">Ten patients completed treatment (two patients discontinued). In 8/10 patients, 89-100% Non-Viable Tissue was removed within 2-8 applications. An average reduction of 35±38% in wound size was measured by the end of the 2-week follow-up period. In 6 patients positive for biofilm at baseline, the biofilm was reduced to single individual or no detected microorganisms by the end of treatment. Red fluorescence (e.g. </span><em><span style="font-family: Raleway; mso-bidi-font-family: Calibri; color: #333333; background: white;">Staphylococcus aureus)</span></em><span style="font-family: 'Times New Roman',serif;"> reduced from 1.52±0.97 cm<sup>2</sup> pre-treatment to 0.64±0.41 cm<sup>2</sup> post treatment. Safety data show that BBD is safe and well tolerated. Biomarker analysis from wound fluid is on-going and results will be presented in the symposium.</span></p><br/><br/><b>Discussion</b>: <p class="Default"><span style="font-size: 11.0pt; mso-fareast-font-family: Calibri; border: none; mso-bidi-language: AR-SA;">The data shows that BBD is safe and can effectively debride wounds and promote wound area reduction while reducing biofilm and bacterial bioburden.</span></p><br/><br/><b>Trademarked Items</b>: <br/><br/><b>References</b>: <br/><br/>

.png)