Case Series/Study
(CS-101) Negative Pressure Wound Therapy with Instillation and Dwell Utilizing a Novel Hybrid Silicone Acrylic Drape Dressing: A Case Series
Methods:
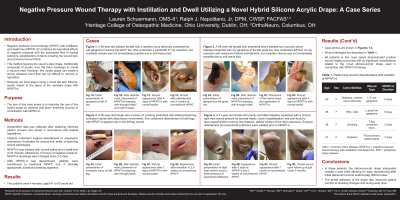
Deidentified data was collected after obtaining informed patient consent and stored in accordance with federal regulations. Patients underwent surgical debridement or amputation intervention if indicated for various foot, ankle, or lower leg wound pathologies followed by NPWTi-d* with normal saline and a dwell time of 20 minutes, followed by 3.5 hours of negative pressure. NPWTi-d dressings were changed every 2-3 days. After NPWTi-d was discontinued, patients were transitioned to traditional NWPT and, if clinically appropriate, advanced dressing regimens.
Results:
The patients were 2 males and 3 females, aged 42 to 61 years old. Wound etiologies included trauma, diabetic foot pathology, lower extremity gangrene, and delayed surgical wound healing. All patients in this case series demonstrated positive wound healing outcomes with no significant complications related to the novel silicone-acrylic drape used in connection with NPWTi-d therapy.
Discussion: In these patients, the silicone-acrylic drape adequately created a seal while allowing for easy repositioning after initial placement around anatomically difficult areas. The gentle adhesion of the drape also improved patient comfort at dressing changes and during wear time.
Trademarked Items: *3M™ Veraflo™ Therapy, §3M™ Dermatac™ Drape; 3M, St. Paul, MN
References:

.png)
