Case Series/Study
(CS-053) Silver delivering Bioresorbable polymer matrix (BPMAg)– as an adjunct to closure in theoretically high risk surgical closures.
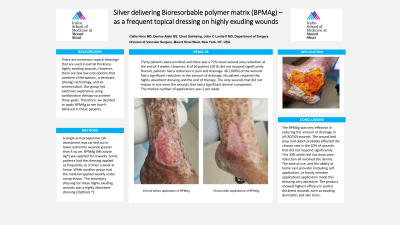
There are many dressings aimed at reducing post operative infection. All of these can deliver a topical antimicrobial or at least have a antimicrobial that is designed to reduce the bacteria in the dressing. The various dressings have not really shown superiority to SOC, with the exception on post operative closed incisional negative pressure wound therapy. With that in mind we undertook a feasibility study to see if the surgical implantation of a silver delivering bioresorbable polymer matrix (BPMAg) may be beneficial.
Methods:
: Twenty four patients undergoing clean operations, but deemed high risk incisions were chosen to have the implantation of BPMAg. This was done as a single arm prospective evaluation. The patients were followed at 4, 14, and 28 days. All patients had the BPMAg placed at the end of the procedure and implanted under the flaps of the wound, the incision was then closed in layers. All patients received pre-incisional gram positive antibiotic coverage.
Results:
None of the patients had a wound infection in the 28 day evaluation period, and none received post operative antibiotics. Operations included; transmetarsal amputations, below knee amputations, groin incisions for EVAR and groin incisions containing graft. This is better than the expected post op wound complication rate of 3% in this population.
Discussion: None of these wounds were deemed contaminated. Of note contaminated wounds would be the most logical place to study such a product. Wounds in which the intestine has been entered, especially colon and rectal resections would be of most interest. This dressing theoretically could reduce bacteria from the inside of the wound, which none of the current topical post operative wound dressings can do. In adition the cost of the product will need to be in line with the proven benefit.
Trademarked Items: BPMAg - is Microlyte Ag not mention by name in the abstract
References:

.png)