Case Series/Study
(CS-086) Temporary abdominal closure (TAC) in open abdomen therapy with negative pressure wound therapy (NPWT): High exudate handling
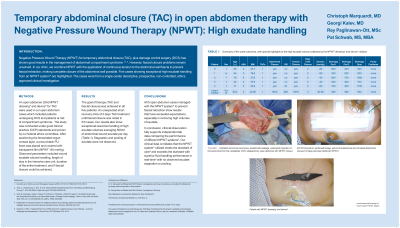
NPWT for temporary abdominal closure (TAC)1,2 has proven results, especially combined with damage control surgery (DCS)3 and for the management of abdominal compartment syndrome [2]. Still the problem of fascial closure remains unsolved. In our clinical practice we combine NPWT with the application of continuous tension to the abdominal wall fascia to prevent fascial retraction, which makes a complete closure of the abdominal wall possible. We enrolled 5 patients in a single-center descriptive, prospective, non-controlled, ethics approved clinical investigation and present these 5 cases that show exceptional high exudate handling from the NPWT system*^.
Methods:
We used an open abdomen (OA) NPWT dressing* and device^ for TAC in our open abdomen cases which included patients undergoing DCS and patients at risk of compartment syndrome. The study was conducted under GCP standards and proven by our federal ethical committee. After positioning the fenestrated organ contact layer, a precut black polyurethane foam and covering with transparent film therapy was set to -80 mmHg. Observed parameters include, wound exudate volume handling, length of stay in the intensive care unit, duration of the entire treatment, and if fascial closure could be achieved.
Results:
The goal of therapy (TAC and fascial closure) was achieved in all five patients. An unexpected short recovery time of 2 days TAC treatment until fascial closure was noted in 5/5 cases. Our results also show exceptional real time handling of high exudate volumes averaging 470 ml of abdominal wound exudate per day. Stagnation and pooling of exudate was not observed.
Discussion:
Our 5 open abdomen cases managed with our NPWT system*^ to prevent fascial retraction have results that have exceeded our expectations, especially in removing high volumes of exudate. In conclusion, clinical observation fully supports independent lab data comparing the performance of different NPWT systems4. Our clinical data correlates that the NPWT system*^ we used meets the standard of care5 and exceeds the standard with superior fluid handling performance in real time4 with no observed exudate stagnation or pooling.
Trademarked Items: Trademarks: Medela, Invia and Invia Liberty are registered in the U.S. Patent and Trademark Office and elsewhere
References: 1. Coccolini et al. World Journal of Emergency Surgery (2018) 13:7 DOI 10.1186/s13017-018-0167-4
2. Töns, C., Schachtrupp, A., Rau, M. et al. Abdominelles Kompartmentsyndrom: Vermeidung und Behandlung. Chirurg 71, 918–926 (2000). https://doi.org/10.1007/s001040051156
3. Sohn M, Iesalnieks I, Agha A, Steiner P, Hochrein A, Pratschke J, Ritschl P, Aigner F. Perforated Diverticulitis with Generalized Peritonitis: Low Stoma Rate Using a "Damage Control Strategy“. World J Surg. 2018 Oct;42(10):3189-3195. doi: 10.1007/s00268-018-4585-y. PMID: 29541823
4. Paglinawan R, Schwab P, Bechert K. Negative pressure wound therapy system Innovates standard of care via intelligent pressure control and dynamic exudate removal. Wounds. 2020;32(10):S1-S8.
5. Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy – overview, challenges and perspectives. J Wound Care. 2017;26(Suppl 3):S1–S113.

.png)