Back
Clinical Research
(CR-005) Treatment of Diabetic Foot Ulcers Using Autologous Adipose Tissue

Co-Author(s):
Zachery Rasor, DPM, AACFAS – Foot and Ankle USA Mid Atlantic; Charles Zelen, DPM – Founder and President, Professional Education and Research Institute (PERI); Ahmed Zobi, BS, EMBA – CEO, Syntr Health Technologies, Inc.
Introduction:
Background: Diabetic foot ulcer (DFU) is a complication of diabetes mellitus (DM) and the leading cause of nontraumatic lower limb amputations. In 2013, an estimated 384 million individuals suffered from DM with a 25% lifetime incidence rate. The etiology of DFU is multifactorial and primarily includes some combination of neuropathy, poor vascularity, and isolated or repeated trauma. Once a DFU has formed, the wound microenvironment is characterized by poor healing due to continued pressure and/or trauma, chronic infections, and displacement of the plantar fat pad. Current DFU treatments are laden with expense and unpredictable outcomes; however, emerging evidence indicates that autologous microsized fat tissue may be a safe and effective alternative to the current treatment options.
Methods:
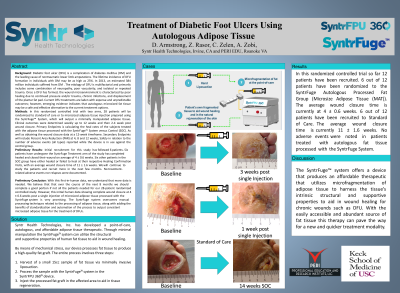
Methods: In this randomized controlled trial with two arms, 28 patients will be randomized to standard of care or to microsized adipose tissue injection prepared using the SyntrFugeTM System, which will output a minimally manipulated adipose tissue. Clinical outcomes were determined weekly up to 12 weeks and then 2-4 week post wound closure. Primary Endpoints is calculating the heal rates of the subjects treated with the adipose tissue processed with the SyntrFuge™ System versus Control (SOC). As well as obtaining the wound closure data at a 12 week timeframe.
Results: Preliminary
Results: Initial recruitment for this study has/is currently following 12 patients. Six patients have undergone the SyntrFuge Treatment arm of the study and have completely healed and closed their wounds, averaging around 3-4 weeks healing time. Some patients have seen complete wound closure in as little as 6 days. Patients in the standard of care (SOC) group have achieved full wound closure averaging around 12 weeks. We will continue to study the patients and recruit more in the next few months.
Discussion: Preliminary
Conclusion: With this first-in-human data, we understand that more data is needed. We believe that that over the course of the next 2-3 months we should complete a good portion if not all the patients needed for our 28 patient randomized controlled study. However, this initial human data showing complete wound closure at 3-4 weeks post a single injection of microsized adipose tissue processed with the SyntrFuge system is very promising. The SyntrFuge system overcomes manual processing techniques related to the processing of adipose tissue, along with adding the benefits of standardization and automation of the process to output consistent microsized adipose tissue for the treatment of DFUs.
Trademarked Items: SyntrFuge System
Syntr Health Technologies, Inc.
SyntrFPU 360
References:
Background: Diabetic foot ulcer (DFU) is a complication of diabetes mellitus (DM) and the leading cause of nontraumatic lower limb amputations. In 2013, an estimated 384 million individuals suffered from DM with a 25% lifetime incidence rate. The etiology of DFU is multifactorial and primarily includes some combination of neuropathy, poor vascularity, and isolated or repeated trauma. Once a DFU has formed, the wound microenvironment is characterized by poor healing due to continued pressure and/or trauma, chronic infections, and displacement of the plantar fat pad. Current DFU treatments are laden with expense and unpredictable outcomes; however, emerging evidence indicates that autologous microsized fat tissue may be a safe and effective alternative to the current treatment options.
Methods:
Methods: In this randomized controlled trial with two arms, 28 patients will be randomized to standard of care or to microsized adipose tissue injection prepared using the SyntrFugeTM System, which will output a minimally manipulated adipose tissue. Clinical outcomes were determined weekly up to 12 weeks and then 2-4 week post wound closure. Primary Endpoints is calculating the heal rates of the subjects treated with the adipose tissue processed with the SyntrFuge™ System versus Control (SOC). As well as obtaining the wound closure data at a 12 week timeframe.
Results: Preliminary
Results: Initial recruitment for this study has/is currently following 12 patients. Six patients have undergone the SyntrFuge Treatment arm of the study and have completely healed and closed their wounds, averaging around 3-4 weeks healing time. Some patients have seen complete wound closure in as little as 6 days. Patients in the standard of care (SOC) group have achieved full wound closure averaging around 12 weeks. We will continue to study the patients and recruit more in the next few months.
Discussion: Preliminary
Conclusion: With this first-in-human data, we understand that more data is needed. We believe that that over the course of the next 2-3 months we should complete a good portion if not all the patients needed for our 28 patient randomized controlled study. However, this initial human data showing complete wound closure at 3-4 weeks post a single injection of microsized adipose tissue processed with the SyntrFuge system is very promising. The SyntrFuge system overcomes manual processing techniques related to the processing of adipose tissue, along with adding the benefits of standardization and automation of the process to output consistent microsized adipose tissue for the treatment of DFUs.
Trademarked Items: SyntrFuge System
Syntr Health Technologies, Inc.
SyntrFPU 360
References:

.png)