Back
Case Series/Study
(CS-010) Novel Closure of a Severe Neonatal Scalp Injury Using Dehydrated Human Amnion Chorion Allograft Matrix

Co-Author(s):
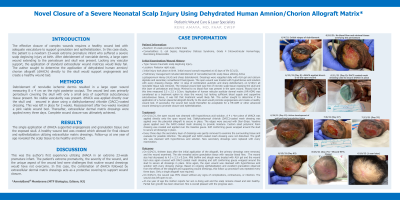
<b>Introduction</b>: The effective closure of complex wounds requires a healthy wound bed with adequate vasculature to support granulation and epithelialization. In this case study, the patient is a newborn 23-week extreme premature infant who suffered a severe scalp degloving injury at birth. After debridement of nonviable dermis, a large open wound extending to the periosteum and skull was present. Lacking any vascular support, the application of standard extracellular wound matrices would likely fail. The author sought to determine if the application of <span class="TrackChangeTextInsertion TrackedChange TrackChangeHoverSelectColorRed SCXW140155835 BCX8"><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun TrackChangeHoverSelectHighlightRed SCXW140155835 BCX8">dehydrated h</span></span></span><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW140155835 BCX8">uman </span></span><span class="TrackChangeTextInsertion TrackedChange SCXW140155835 BCX8"><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW140155835 BCX8">amnion/chorion a</span></span></span><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW140155835 BCX8">llograft </span></span><span class="TrackChangeTextInsertion TrackedChange SCXW140155835 BCX8"><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW140155835 BCX8">(</span></span></span><span class="TrackChangeTextInsertion TrackedChange SCXW140155835 BCX8"><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SpellingErrorV2Themed SCXW140155835 BCX8">dHACA</span></span></span><span class="TrackChangeTextInsertion TrackedChange SCXW140155835 BCX8"><span class="TextRun SCXW140155835 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW140155835 BCX8">) </span></span></span> directly to the skull would support angiogenesis and create a healthy wound bed.<br/><br/><b>Methods</b>: Debridement of nonviable ischemic dermis resulted in a large open wound measuring 8 x 4 cm on the right posterior occiput. The wound bed was primarily periosteum covering the skull with only a small area of superficial subcutaneous tissue present. No visible vascularity was present. <span class="TextRun SCXW217020228 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SpellingErrorV2Themed SpellingErrorHighlight SCXW217020228 BCX8">dHACA</span></span> was applied directly over the skull and secured in place using a dialkylcarbamoyl chloride (DACC)-coated dressing. This was left in place for 2 weeks. Reassessment after two weeks revealed a pink viable wound bed. Thereafter, extracellular dermal matrix dressings were applied every three days. Complete wound closure was ultimately achieved.<br/><br/><b>Results</b>: The single application of <span class="TextRun SCXW35450468 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SpellingErrorV2Themed SpellingErrorHighlight SCXW35450468 BCX8">dHACA</span></span> supported angiogenesis and granulation tissue over the exposed skull. A healthy wound bed was created which allowed for final closure and epithelialization utilizing extracellular matrix dressings. <span class="TrackChangeTextInsertion TrackedChange SCXW193465143 BCX8"><span class="TextRun SCXW193465143 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW193465143 BCX8">Follow-up at one year of age reveal</span></span></span><span class="TrackChangeTextInsertion TrackedChange SCXW193465143 BCX8"><span class="TextRun SCXW193465143 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW193465143 BCX8">ed</span></span></span><span class="TrackChangeTextInsertion TrackedChange SCXW193465143 BCX8"><span class="TextRun SCXW193465143 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SCXW193465143 BCX8"> the scalp tissue to be healthy and intact.</span></span></span><span class="EOP SCXW193465143 BCX8" data-ccp-props="{"201341983":0,"335551550":6,"335551620":6,"335559739":0,"335559740":240}"> </span><br/><br/><b>Discussion</b>: This was the author's first experience utilizing <span class="TextRun SCXW104423421 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SpellingErrorV2Themed SpellingErrorHighlight SCXW104423421 BCX8">dHACA</span></span> in an extreme 23-week premature infant. The patient's extreme prematurity, the severity of the wound, and the unique aspect of the wound bed were challenges that routine wound dressings would have not overcome. In this case, the combination of <span class="TextRun SCXW104423421 BCX8" lang="EN-US" xml:lang="EN-US" data-contrast="auto"><span class="NormalTextRun SpellingErrorV2Themed SpellingErrorHighlight SCXW104423421 BCX8">dHACA</span></span> followed by extracellular dermal matrix dressings proved to be effective and safe.<br/><br/><b>Trademarked Items</b>: AmnioBand®<br/><br/><b>References</b>: <br/><br/>

.png)