Back
Purpose: Multivitamin tablets containing iron salts are often formulated with ascorbic acid to improve the absorption of iron.1 However, formulations containing iron salts with ascorbic acid can form unsightly black spots (considered harmless) in the presence of moisture. A pigmented film coating system can conceal and reduce the visual impact of these unsightly dark spots and improve consumer adherence. In this study multivitamin tablets, with and without a range of different film coatings, were stored at ambient and accelerated ICH conditions for 3 – 6 months to understand the impact of the film coating system on the ability to visualize the dark spots.
Methods: 1000 mg multivitamin tablets were coated with Opadry®, Opadry II 85 series, Opadry QX, Opadry amb, or Opadry amb II film coating systems to a 4% weight gain (WG) in a Labcoat I (O’Hara Technologies, Inc.). The coated tablets were stored in induction sealed 75 mL HDPE bottles for 3 months at 40°C / 75% RH and up to 6 months at 30°C / 65% RH. Uncoated tablets were also exposed to 40°C / 75% RH open dish conditions for 24 hours to understand the maximum potential for dark spots. Following storage, tablets were examined for dark spots and color changes, and images were taken for comparative purposes. Tablet color was measured analytically using a DataColor600 (DataColor, Inc.) with Limit of CIE LAB total color difference (DE) defined as 1.5 for white samples.
Results: Uncoated 1000mg multivitamin tablets exposed to 40°C / 75% RH open dish conditions for 24 hours exhibited dark brown spots, which are shown in Figure 1. 1000 mg multivitamin tablets stored in sealed HDPE bottles in both ambient (30°C / 65% RH) and accelerated (40°C / 75% RH) conditions were examined for the appearance of brown spots on the surface of the tablets and color change. The appearance results are shown in Figure 2. Tablets coated with Opadry or Opadry amb II did not show any brown spots when stored at either condition, providing a very consistent appearance. When coated on multivitamin tablets, Opadry amb, Opadry II 85 series, and Opadry QX exhibited a change in appearance. Tablets coated with Opadry amb exhibited slight yellowing at 40C / 75% RH, while tablets coated with Opadry II 85 series and Opadry QX exhibited small but noticeable brown spots at 30C / 65% RH and distinct brown spots at 40C / 75% RH. Color difference measurements also confirmed a significant color change for multivitamin tablets coated with Opadry II 85 series and Opadry QX stored at both ambient and accelerated conditions, as confirmed by DE values greater than 1.5. In comparison, Opadry and Opadry amb II coated tablets did not have a significant color change in either condition.
Conclusion: Multivitamin tablets are prone to form unsightly brown spots when stored under accelerated conditions. A pigmented film coating system with hiding power is an effective way to reduce the visual impact of these unsightly dark spots. However, not all coatings provide the same level of hiding power to cover color changes in the core. By selecting the right coating solution for a particular active unsightly visual defects can be avoided, and a consistent color and appearance can be ensured. For this application Opadry or Opadry amb, film coating systems gave the best results and would be the recommended solutions.
References: 1. Teucher B, Olivares M, Cori H. Enhancers of Iron Absorption: Ascorbic Acid and other Organic Acids. International Journal Name of Vitamin Nutrition Research. 2004;74(6): 403-419.
.jpg)
Uncoated Multivitamin Tablet Before and After Storage for 24 hours at 40°C / 75% RH in Open Dish Conditions.
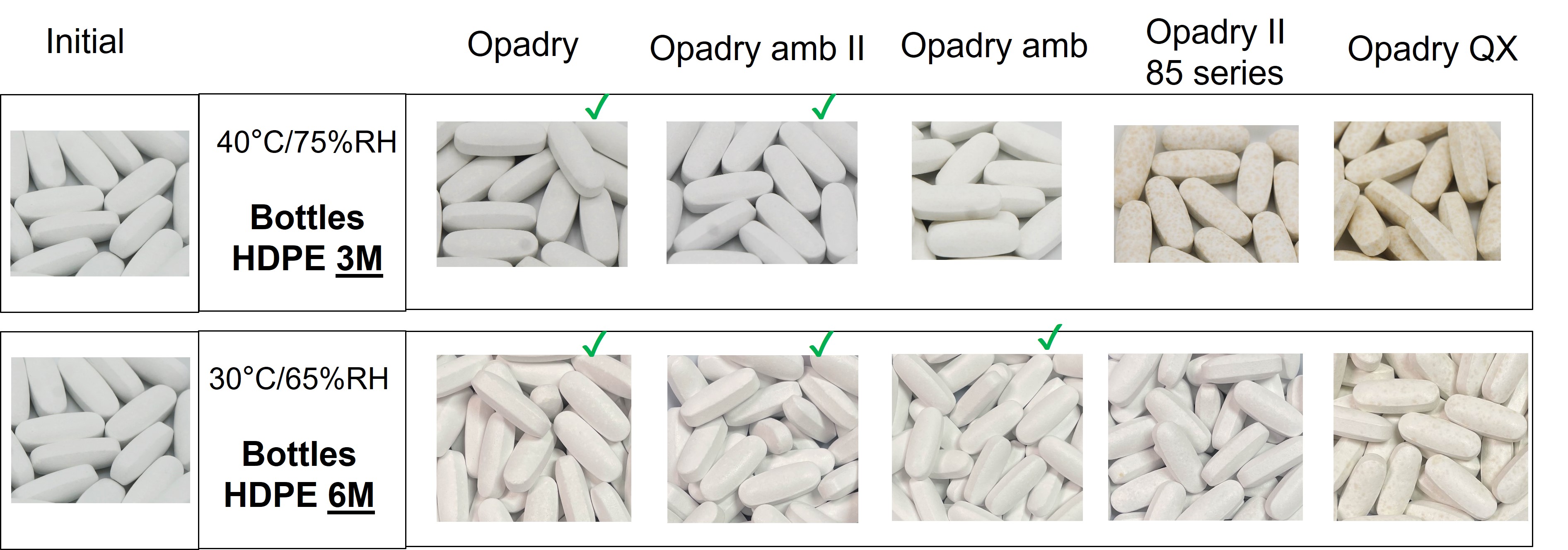
Comparison of Coated Multivitamin Tablet Before and After Storage for 3M at 40°C / 75% RH and 6M 30°C / 65% RH
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(T1230-07-39) Application of Film Coating Systems to Hide Dark Spots on Multivitamin Tablets
Tuesday, October 18, 2022
12:30 PM – 1:30 PM ET
- DT
Daniel To, Ph.D.
Colorcon, Inc.
Harleysville, Pennsylvania, United States - jH
john Horan
Colorcon, Inc.
Harleysville, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Purpose: Multivitamin tablets containing iron salts are often formulated with ascorbic acid to improve the absorption of iron.1 However, formulations containing iron salts with ascorbic acid can form unsightly black spots (considered harmless) in the presence of moisture. A pigmented film coating system can conceal and reduce the visual impact of these unsightly dark spots and improve consumer adherence. In this study multivitamin tablets, with and without a range of different film coatings, were stored at ambient and accelerated ICH conditions for 3 – 6 months to understand the impact of the film coating system on the ability to visualize the dark spots.
Methods: 1000 mg multivitamin tablets were coated with Opadry®, Opadry II 85 series, Opadry QX, Opadry amb, or Opadry amb II film coating systems to a 4% weight gain (WG) in a Labcoat I (O’Hara Technologies, Inc.). The coated tablets were stored in induction sealed 75 mL HDPE bottles for 3 months at 40°C / 75% RH and up to 6 months at 30°C / 65% RH. Uncoated tablets were also exposed to 40°C / 75% RH open dish conditions for 24 hours to understand the maximum potential for dark spots. Following storage, tablets were examined for dark spots and color changes, and images were taken for comparative purposes. Tablet color was measured analytically using a DataColor600 (DataColor, Inc.) with Limit of CIE LAB total color difference (DE) defined as 1.5 for white samples.
Results: Uncoated 1000mg multivitamin tablets exposed to 40°C / 75% RH open dish conditions for 24 hours exhibited dark brown spots, which are shown in Figure 1. 1000 mg multivitamin tablets stored in sealed HDPE bottles in both ambient (30°C / 65% RH) and accelerated (40°C / 75% RH) conditions were examined for the appearance of brown spots on the surface of the tablets and color change. The appearance results are shown in Figure 2. Tablets coated with Opadry or Opadry amb II did not show any brown spots when stored at either condition, providing a very consistent appearance. When coated on multivitamin tablets, Opadry amb, Opadry II 85 series, and Opadry QX exhibited a change in appearance. Tablets coated with Opadry amb exhibited slight yellowing at 40C / 75% RH, while tablets coated with Opadry II 85 series and Opadry QX exhibited small but noticeable brown spots at 30C / 65% RH and distinct brown spots at 40C / 75% RH. Color difference measurements also confirmed a significant color change for multivitamin tablets coated with Opadry II 85 series and Opadry QX stored at both ambient and accelerated conditions, as confirmed by DE values greater than 1.5. In comparison, Opadry and Opadry amb II coated tablets did not have a significant color change in either condition.
Conclusion: Multivitamin tablets are prone to form unsightly brown spots when stored under accelerated conditions. A pigmented film coating system with hiding power is an effective way to reduce the visual impact of these unsightly dark spots. However, not all coatings provide the same level of hiding power to cover color changes in the core. By selecting the right coating solution for a particular active unsightly visual defects can be avoided, and a consistent color and appearance can be ensured. For this application Opadry or Opadry amb, film coating systems gave the best results and would be the recommended solutions.
References: 1. Teucher B, Olivares M, Cori H. Enhancers of Iron Absorption: Ascorbic Acid and other Organic Acids. International Journal Name of Vitamin Nutrition Research. 2004;74(6): 403-419.
.jpg)
Uncoated Multivitamin Tablet Before and After Storage for 24 hours at 40°C / 75% RH in Open Dish Conditions.

Comparison of Coated Multivitamin Tablet Before and After Storage for 3M at 40°C / 75% RH and 6M 30°C / 65% RH
