Back
Purpose: Developing a formulation for oral delivery of pharmaceutical compounds with low dissolution rate and poor aqueous solubility may be challenging for toxicology evaluation at high doses. Amorphous Solid Dispersions (ASD) are often introduced into early drug development as one of the most effective techniques to improve solubility and facilitate oral absorption. In the ASD, the crystalline drug is converted into an amorphous drug stabilized by a carrier (usually a polymer) to form a matrix. The composition of the ASD, including type of polymer and drug load, is known to play an important role in the performance of the formulation, where different drug loads often affect bioavailability. The goal of this work was to evaluate the most suitable drug load for an amorphous spray dry dispersion of a compound (GEN-A1) with challenging physicochemical properties. In-vitro dissolution tools were applied to characterize ASD at different drug loads and to provide insight of potential formulation performance.
Methods: The amorphous solid dispersions of compound GEN-A1 were prepared using a spray drying process with a benchtop spray dryer Buchi B290. Based on API solubility in spray organic solvents and in-vivo target dose, the drug: polymer ratio in the composition was explored at 20:80 (low drug load), 30:70 (mid drug load) and 40:60 (high drug load). HPMCAS-MF was selected as the best solution stabilizer from amorphous solubility measurements in the presence of different polymers. Optimized pre-set spray dry parameters were used for best outcome (yields and consistent PSD). After secondary drying, the dispersions were characterized by pXRD, SEM, PLM, mDSC, PSD and in-vitro performance (FaSSiF-V2 dissolution using µDisso) was also assessed. The three different ASDs were formulated and dosed in an in-vivo oral PK rat study at a dose of 300 mg/Kg to evaluate the impact of the drug load and coordinate with the in-vitro performance.
Results: The ASD in-vitro dissolution data correlated well with the observed pharmacokinetic performance in rodents. Although all ASD were amorphous and similar in PSD, the dissolution studies with μDisso revealed a consistent decreasing rate of drug release with increasing drug load (Figure 1). Interestingly, the in-vivo data also reflected the same findings. Among three different drug: polymer compositions, 20:80 showed the highest AUC last (hr*umol/L), followed by the 30:70 composition with the 40:60 giving the lowest. All ASD in-vivo exposures were higher than the crystalline API (Figure 2).
Conclusion: This work demonstrates the use of preclinical bench-top dissolution assessment to guide the selection of drug loading in amorphous solid dispersions for optimal bioperformance in-vivo. The µDisso method demonstrated a good correlation with the in-vivo data to predict the behavior of the amorphous solid dispersion at different drug: polymer compositions. The µDisso is a valid tool to limit the in-vivo testing when selecting an ASD formulation and could be further optimized into smaller scale assays.
References: 1. In Vitro and In Vivo Behaviors of KinetiSol and Spray-Dried Amorphous Solid Dispersions of a Weakly Basic Drug and Ionic Polymer; Scott V. Jermain, Michael B. Lowinger, Daniel J. Ellenberger, Dave A. Miller, Yongchao Su, and Robert O. Williams; Molecular Pharmaceutics 2020 17 (8), 2789-2808
2. Processing Impact on In Vitro and In Vivo Performance of Solid Dispersions—A Comparison between Hot-Melt Extrusion and Spray Drying. Li, Y.; Mann, A.K.P.; Zhang, D.; Yang, Z.; Pharmaceutics 2021, 13, 1307.
3. A pH-Dilution Method for Estimation of Biorelevant Drug Solubility along the Gastrointestinal Tract: Application to Physiologically Based Pharmacokinetic Modeling; Yi Gao, Robert A. Carr, Julie K. Spence, Weili W. Wang, Teresa M. Turner, John M. Lipari, and Jonathan M. Miller; Molecular Pharmaceutics 2010 7 (5), 1516-1526
4. Ritonavir-PEG 8000 amorphous solid dispersions: in vitro and in vivo evaluations. Devalina Law 1, Eric A Schmitt, Kennan C Marsh, Elizabeth A Everitt, Weili Wang, James J Fort, Steven L Krill, Yihong Qiu; J Pharm Sci. 2004 Mar;93(3):563-70
Acknowledgments: Small Molecule Pharmaceutical Science, Genentech, Inc.
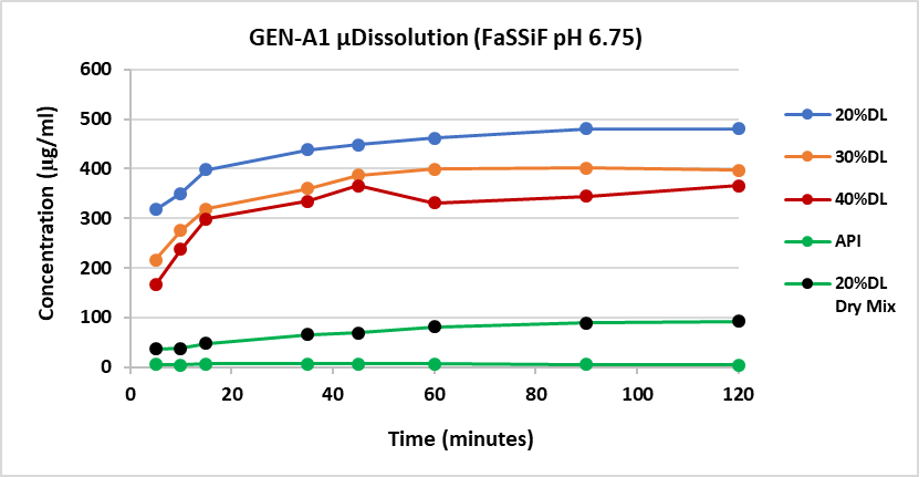
Figure 1: Dissolution release profile of ASD: 20% drug load, 30% drug load, 40% drug load, crystalline API and dry mix of 20% API with HPMCAS-MF in FaSSIF-v2 pH6.75 over 120 minutes using Pion µDissolution.
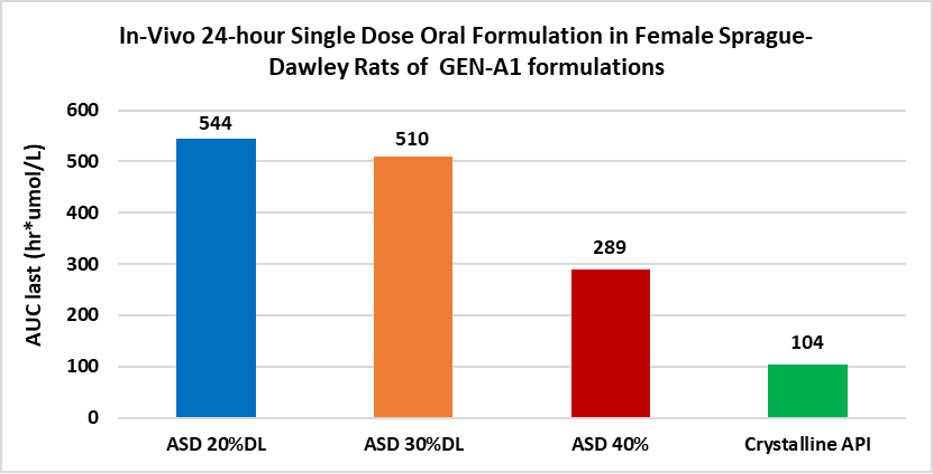
Figure 2: In-vivo PK data at 300mg/Kg in rat with ASD at 20% drug load, 30% drug load and 40% drug load in 2% HPC (Hydroxypropylcellulose) in water vs. Crystalline formulation in MCT (0.6% w/v Methylcellulose, 0.2% w/v Tween 80 in water)
Discovery and Basic Research - Pharmaceutics
Category: Late Breaking Poster Abstract
(M1230-10-57) Correlating In Vitro and In Vivo Performance of Low-to-High Drug Load Amorphous Spray-Dry Dispersions
Monday, October 17, 2022
12:30 PM – 1:30 PM ET
- LA
Le An, BS
Genentech, Inc.
South San Francisco, California, United States - LA
Le An, BS
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Purpose: Developing a formulation for oral delivery of pharmaceutical compounds with low dissolution rate and poor aqueous solubility may be challenging for toxicology evaluation at high doses. Amorphous Solid Dispersions (ASD) are often introduced into early drug development as one of the most effective techniques to improve solubility and facilitate oral absorption. In the ASD, the crystalline drug is converted into an amorphous drug stabilized by a carrier (usually a polymer) to form a matrix. The composition of the ASD, including type of polymer and drug load, is known to play an important role in the performance of the formulation, where different drug loads often affect bioavailability. The goal of this work was to evaluate the most suitable drug load for an amorphous spray dry dispersion of a compound (GEN-A1) with challenging physicochemical properties. In-vitro dissolution tools were applied to characterize ASD at different drug loads and to provide insight of potential formulation performance.
Methods: The amorphous solid dispersions of compound GEN-A1 were prepared using a spray drying process with a benchtop spray dryer Buchi B290. Based on API solubility in spray organic solvents and in-vivo target dose, the drug: polymer ratio in the composition was explored at 20:80 (low drug load), 30:70 (mid drug load) and 40:60 (high drug load). HPMCAS-MF was selected as the best solution stabilizer from amorphous solubility measurements in the presence of different polymers. Optimized pre-set spray dry parameters were used for best outcome (yields and consistent PSD). After secondary drying, the dispersions were characterized by pXRD, SEM, PLM, mDSC, PSD and in-vitro performance (FaSSiF-V2 dissolution using µDisso) was also assessed. The three different ASDs were formulated and dosed in an in-vivo oral PK rat study at a dose of 300 mg/Kg to evaluate the impact of the drug load and coordinate with the in-vitro performance.
Results: The ASD in-vitro dissolution data correlated well with the observed pharmacokinetic performance in rodents. Although all ASD were amorphous and similar in PSD, the dissolution studies with μDisso revealed a consistent decreasing rate of drug release with increasing drug load (Figure 1). Interestingly, the in-vivo data also reflected the same findings. Among three different drug: polymer compositions, 20:80 showed the highest AUC last (hr*umol/L), followed by the 30:70 composition with the 40:60 giving the lowest. All ASD in-vivo exposures were higher than the crystalline API (Figure 2).
Conclusion: This work demonstrates the use of preclinical bench-top dissolution assessment to guide the selection of drug loading in amorphous solid dispersions for optimal bioperformance in-vivo. The µDisso method demonstrated a good correlation with the in-vivo data to predict the behavior of the amorphous solid dispersion at different drug: polymer compositions. The µDisso is a valid tool to limit the in-vivo testing when selecting an ASD formulation and could be further optimized into smaller scale assays.
References: 1. In Vitro and In Vivo Behaviors of KinetiSol and Spray-Dried Amorphous Solid Dispersions of a Weakly Basic Drug and Ionic Polymer; Scott V. Jermain, Michael B. Lowinger, Daniel J. Ellenberger, Dave A. Miller, Yongchao Su, and Robert O. Williams; Molecular Pharmaceutics 2020 17 (8), 2789-2808
2. Processing Impact on In Vitro and In Vivo Performance of Solid Dispersions—A Comparison between Hot-Melt Extrusion and Spray Drying. Li, Y.; Mann, A.K.P.; Zhang, D.; Yang, Z.; Pharmaceutics 2021, 13, 1307.
3. A pH-Dilution Method for Estimation of Biorelevant Drug Solubility along the Gastrointestinal Tract: Application to Physiologically Based Pharmacokinetic Modeling; Yi Gao, Robert A. Carr, Julie K. Spence, Weili W. Wang, Teresa M. Turner, John M. Lipari, and Jonathan M. Miller; Molecular Pharmaceutics 2010 7 (5), 1516-1526
4. Ritonavir-PEG 8000 amorphous solid dispersions: in vitro and in vivo evaluations. Devalina Law 1, Eric A Schmitt, Kennan C Marsh, Elizabeth A Everitt, Weili Wang, James J Fort, Steven L Krill, Yihong Qiu; J Pharm Sci. 2004 Mar;93(3):563-70
Acknowledgments: Small Molecule Pharmaceutical Science, Genentech, Inc.

Figure 1: Dissolution release profile of ASD: 20% drug load, 30% drug load, 40% drug load, crystalline API and dry mix of 20% API with HPMCAS-MF in FaSSIF-v2 pH6.75 over 120 minutes using Pion µDissolution.

Figure 2: In-vivo PK data at 300mg/Kg in rat with ASD at 20% drug load, 30% drug load and 40% drug load in 2% HPC (Hydroxypropylcellulose) in water vs. Crystalline formulation in MCT (0.6% w/v Methylcellulose, 0.2% w/v Tween 80 in water)
