Back
Purpose: With the increase in the popularity of drug combinations, the overall goal of our project was to design a formulation platform strategy for the dissolution enhancement of two APIs. The first step in this process would be to exploit the potential interaction between the two APIs to stabilize one another. The second step would be to add a polymer that can selectively interact with the “excess” drug and stabilize it in the amorphous state. Sulfamethoxazole (SMZ) and trimethoprim (TMP) were used as model drugs to design amorphous solid dispersions (ASDs) for the simultaneous solubility enhancement of two active pharmaceutical ingredients (APIs).
Methods: SMZ and TMP at weight ratios of 5:1 and 1:5 were formulated into ternary ASDs with an aminoalkyl methacrylate copolymer [Eudragit® E (EDE)], and polyacrylic acid (PAA), respectively. The ASDs were characterized by thermal (differential scanning calorimetry, DSC), spectroscopic (dielectric (DES), IR and NMR) and X-ray diffractometric (XRD) techniques. The dissolution performance was evaluated under non-sink conditions.
Results: The drug-drug and drug-polymer interactions were characterized to be ionic by IR and solid-state NMR. The interactions resulted in a substantial reduction in molecular mobility, evident from the increase in relaxation time determined by DES. The strength of intermolecular interactions was also estimated from the glass transition temperatures of the ASDs obtained by DSC. The strong intermolecular interactions resulted in stable ASDs with no evidence of crystallization under accelerated stability conditions (elevated temperatures; 40°C/75% relative humidity). SMZ and TMP, in their ternary ASDs, exhibited respectively a 6.4- and 4.6-fold higher area under the curve (AUC) in their concentration time profiles, compared with their corresponding crystalline APIs. Importantly, synchronized release of the two drugs was observed.
Conclusion: The drug-drug and drug-polymer interactions improved the physical stability of the ternary ASDs by reducing molecular mobility. The proposed ASD design strategy could be a potential approach for the solubility enhancement of drug combinations with poor aqueous solubility. A single-phase ternary ASD, stabilized by strong intermolecular interactions, is likely responsible for the unique synchronized release profile.
Acknowledgements: This project was supported by the William and Mildred Peters endowment fund. The authors acknowledge Drs. Dabing Chen and Li Zhong from the Material and Analytical Sciences Department, Boehringer Ingelheim, for their help with the dissolution and HPLC study.
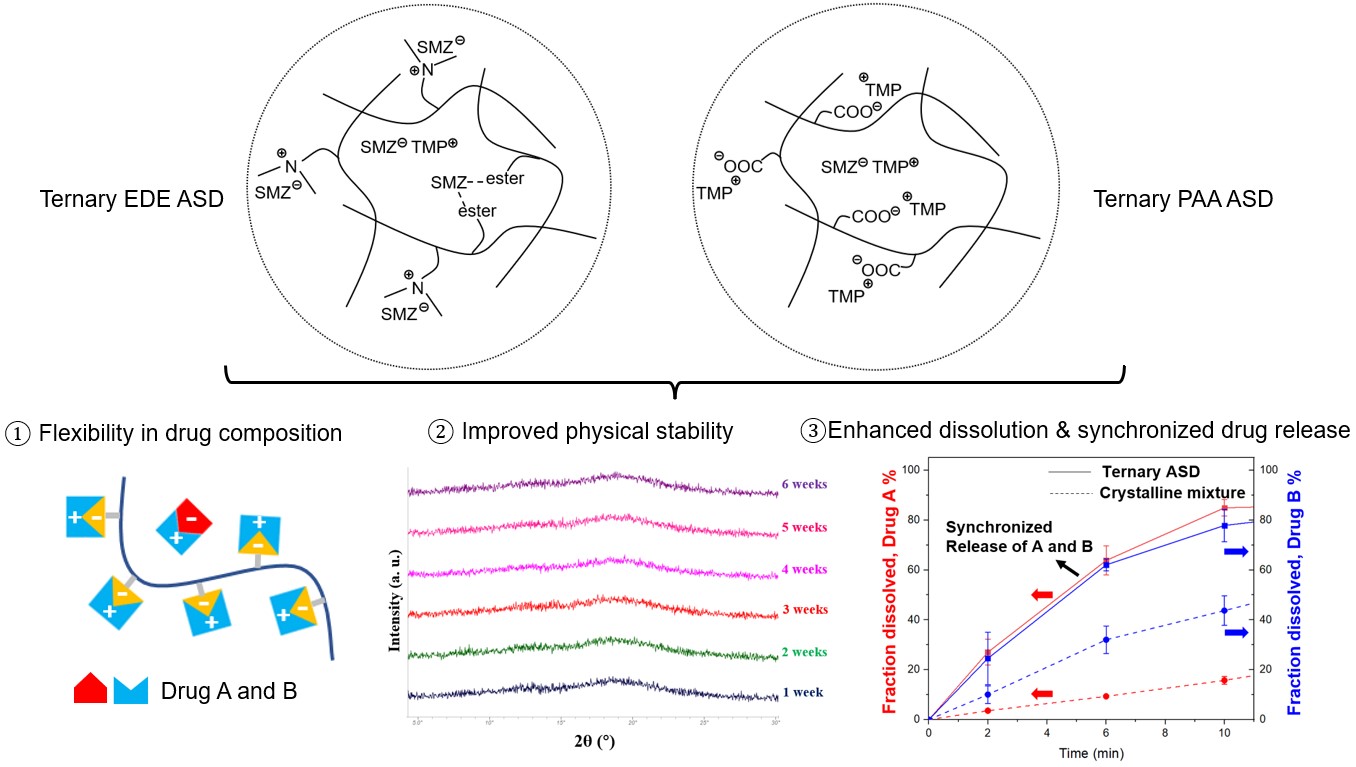
Graphic Abstract
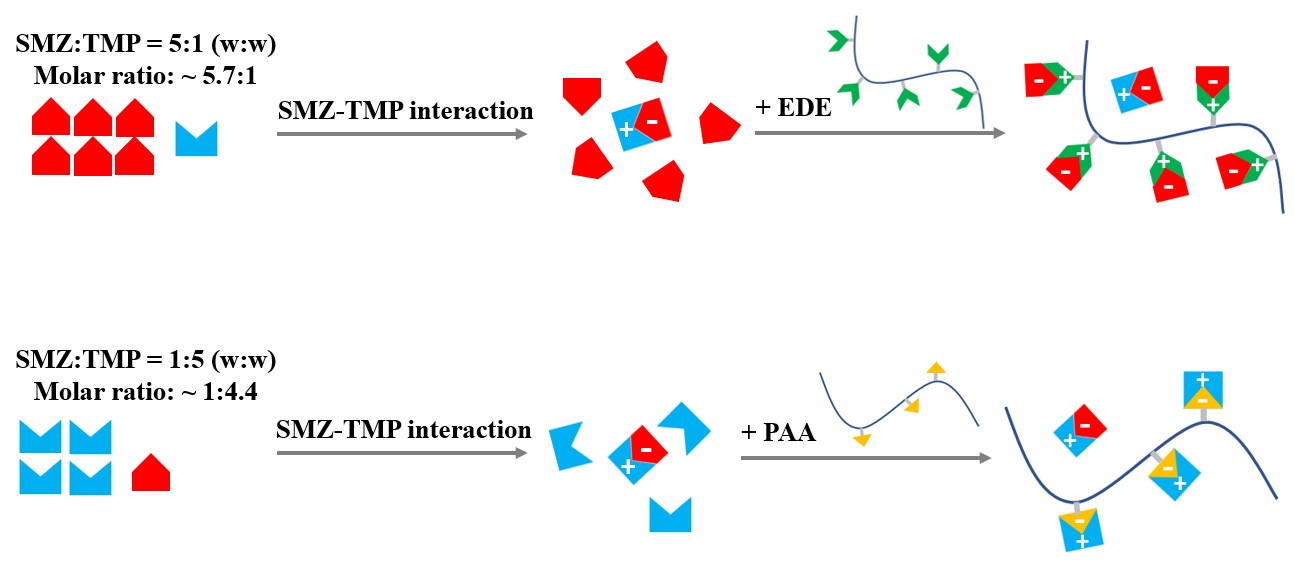
Schematic of the design approach for ternary ASD. Step 1: SMZ-TMP interaction in the amorphous state. Step 2: introducing polymer to stabilize the “excess” component by drug-polymer interaction.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W1030-04-21) Design of Amorphous Solid Dispersions for the Synchronized Release of Two Drugs
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- JL
Jinghan LI, MS
University of Minnes
Minneapolis, Minnesota, United States - JL
Jinghan LI, MS
University of Minnes
Minneapolis, Minnesota, United States
Presenting Author(s)
Main Author(s)
Purpose: With the increase in the popularity of drug combinations, the overall goal of our project was to design a formulation platform strategy for the dissolution enhancement of two APIs. The first step in this process would be to exploit the potential interaction between the two APIs to stabilize one another. The second step would be to add a polymer that can selectively interact with the “excess” drug and stabilize it in the amorphous state. Sulfamethoxazole (SMZ) and trimethoprim (TMP) were used as model drugs to design amorphous solid dispersions (ASDs) for the simultaneous solubility enhancement of two active pharmaceutical ingredients (APIs).
Methods: SMZ and TMP at weight ratios of 5:1 and 1:5 were formulated into ternary ASDs with an aminoalkyl methacrylate copolymer [Eudragit® E (EDE)], and polyacrylic acid (PAA), respectively. The ASDs were characterized by thermal (differential scanning calorimetry, DSC), spectroscopic (dielectric (DES), IR and NMR) and X-ray diffractometric (XRD) techniques. The dissolution performance was evaluated under non-sink conditions.
Results: The drug-drug and drug-polymer interactions were characterized to be ionic by IR and solid-state NMR. The interactions resulted in a substantial reduction in molecular mobility, evident from the increase in relaxation time determined by DES. The strength of intermolecular interactions was also estimated from the glass transition temperatures of the ASDs obtained by DSC. The strong intermolecular interactions resulted in stable ASDs with no evidence of crystallization under accelerated stability conditions (elevated temperatures; 40°C/75% relative humidity). SMZ and TMP, in their ternary ASDs, exhibited respectively a 6.4- and 4.6-fold higher area under the curve (AUC) in their concentration time profiles, compared with their corresponding crystalline APIs. Importantly, synchronized release of the two drugs was observed.
Conclusion: The drug-drug and drug-polymer interactions improved the physical stability of the ternary ASDs by reducing molecular mobility. The proposed ASD design strategy could be a potential approach for the solubility enhancement of drug combinations with poor aqueous solubility. A single-phase ternary ASD, stabilized by strong intermolecular interactions, is likely responsible for the unique synchronized release profile.
Acknowledgements: This project was supported by the William and Mildred Peters endowment fund. The authors acknowledge Drs. Dabing Chen and Li Zhong from the Material and Analytical Sciences Department, Boehringer Ingelheim, for their help with the dissolution and HPLC study.

Graphic Abstract

Schematic of the design approach for ternary ASD. Step 1: SMZ-TMP interaction in the amorphous state. Step 2: introducing polymer to stabilize the “excess” component by drug-polymer interaction.
