Back
Purpose: Bacteria can communicate via different signals or inducers. Autoinducer molecules serve as a common communicator or ‘signal’ of both gram positive and negative bacteria. Once this ‘signal’ reaches a threshold, this leads to cumulative dendritic cell activation. This activation allows the human body to initiate an innate immune response, and eventually an adaptive response to the foreign invader. Autoinducer-1 (AI-1) molecule, N-octanoyl-L-Homoserine lactone, serves as a common communicator between both types of bacteria. This molecule is a member of the N-acyl-homoserine lactone family. Previously, DPD or autoinducer-2, was determined immunogenic and had adjuvant potential1. Since the AI-1 compound is a common ‘signal’ for bacterial communication, this compound can be investigated to determine its adjuvant potency in vaccines. Converting this compound into a microparticle allows this compound to appear even more foreign to the body, and thus, mounting a stronger immune response. There are currently only a few FDA approved adjuvants. Not all FDA approved adjuvants work 100% of the time in vaccine formulations of which the primary function is to increase the immune response. Thus, more adjuvants are needed to couple with vaccines that can prevent bacterial and viral infections. We studied the immunological profile of this microparticulate compound and its potential for serving as an adjuvant in vaccine formulations.
Methods: The AI-1 microparticles (MPs) were formulated by a W/O/W double-emulsion solvent evaporation method by using the PLGA (poly (lactic-co-glycolic acid) polymer. This emulsion was then lyophilized. Two antigens, Colonization Factor Antigen I (CFA/I) from E. Coli. and the inactive Protective Antigen (PA) from Bacillus anthracis, were formulated with Bovine Serum Albumin (BSA) polymer. The two antigens were mixed with the crosslinked-albumin matrix and spray dried using Buchi mini spray dryer B-290. Other FDA approved adjuvants: Alum, MF59, and CpG were formulated via spray drying. The MPs were characterized for particle size, charge, and the polydispersity index. The surface morphology was visualized by scanning electron microscopy. In vitro immunogenicity or the innate response was observed via Griess’s assay by measuring the release of nitric oxide (NO) by dendritic cells which is an antigen presenting cell (APC). NO was measured by comparing AI-1 and the FDA approved adjuvants alone and when paired with both antigens (CFA and PA). Autophagy plays an important role in antigen presentation. Hence, the ability of vaccine MP to induce autophagosomes in murine dendritic cells was evaluated using CYTO-ID® Autophagy detection kit. Measurement of encapsulation efficiency and in vitro release of encapsulated protein from microparticles was done via the bicinchoninic acid (BCA) assay. Cytotoxicity was measured via the MTT assay (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide). Adjuvant potential was evaluated by paring adjuvant AI-1 MPs with particulate vaccines for measles, zika virus, influenza, and meningococcal polysaccharide. Griess’s assay analyzed the particulate vaccines with and without combination with different particulate adjuvants as Alum, Addavax (MF59), MPL-A and CpG.
Results: The MP were characterized for particle size, charge, and the polydispersity index: 4.43±0.29 um, -32.0±0.92 mV, and 0.468. The low PDI indicates uniform size distribution of the particles. The negative surface charge indicates the high stability of MP in an aqueous solution. Morphologically, the particles were found to be spherical. Spherical shape indicates better antigen uptake by the Antigen Presenting Cells (APCs). The release of nitric oxide by dendritic cells was comparable to the FDA approved adjuvants. NO release was found to be higher of CFA antigen and AI-1 adjuvant verses the antigen alone. MPs were found to be non-cytotoxic to the dendritic cells. Adjuvant potential was evaluated by paring the adjuvant AI-1 with particulate vaccines. Adjuvanted Vaccine MP (AI-1) showed a comparable NO release versus the FDA approved adjuvants indicating that AI-1 is immunogenic. NO release was significantly higher when AI-1 was paired with particulate vaccines for measles, influenza, and meningococcal polysaccharide, but not for zika virus indicating AI-1 does have adjutancy potential. BCA assay was used to determine encapsulation efficiency and percent release of encapsulated adjuvant from microparticles. Encapsulation efficiency ranged from 51-70%. Approximately 40 % of the encapsulated adjuvant was released within 24 hours indicating that PLGA has an extended-release pattern.
Conclusion: The microparticulate formulations of AI-1 and all other MPs are non-cytotoxic towards the dendritic cells. AI-1 is immunogenic as the FDA approved adjuvants such as Alum, MF59, and CpG. CFA antigen and AI-1 adjuvants yielded better NO release from dendric cells versus PA antigen and AI-1 pair indicating that AI-1 may not work with all antigens. Additional studies with different antigens is needed to have a better understanding of what AI-1 adjuvant works well with. Adjutancy potential was seen when AI-1 was paired with several particulate vaccines indicating that AI-1 can be used for both bacterial and viral infections. More studies are needed with greater number of vaccine candidates and an in vivo study can confirm the adjutancy potential of autoinducer-1.
References: Joshi D, Chbib C, Uddin MN, D'Souza MJ. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021 Jun 15;23(4):84. doi: 10.1208/s12248-021-00617-6. PMID: 34131810.
Acknowledgements: Colonization Factor Antigen I (CFA I) and Protective Antigen (PA) were obtained from BEI resources.
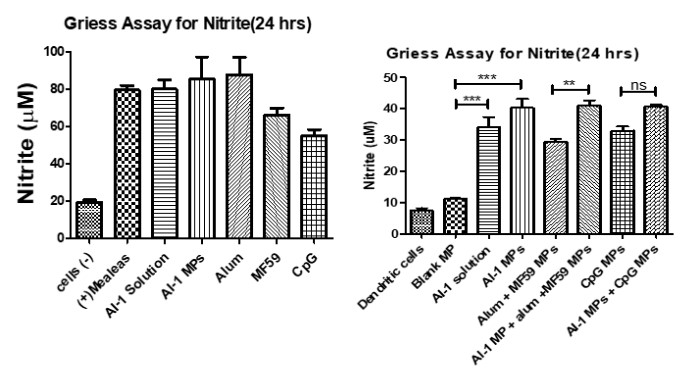
Nitrite oxide release from dendritic cells after stimulation for 24 hrs. Autoinder-1 (AI-1) compared versus the FDA approved adjuvants: Alum, MF59, and CpG. NO release was compared when AI-1 was paired with other adjuvants to observe combination effect. Release of NO was significantly higher when AI-1 was paired with alum, MF59, and CpG MPs.
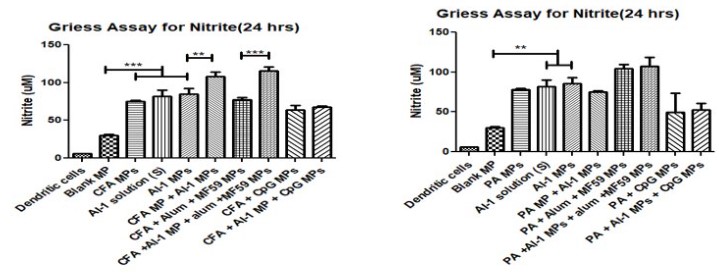
Release of nitric oxide by dendritic cells was measured when AI-1 MP was paired with two antigens. The first antigen is Colonization Factor Antigen I MP (CFA/I) from E. Coli and the second is Protective Antigen (PA) MP from inactivated Bacillus anthracis. AI-1 MP when paired with CFA MP and PA MP antigen, displayed significantly higher NO release when compared to the blank MPs.
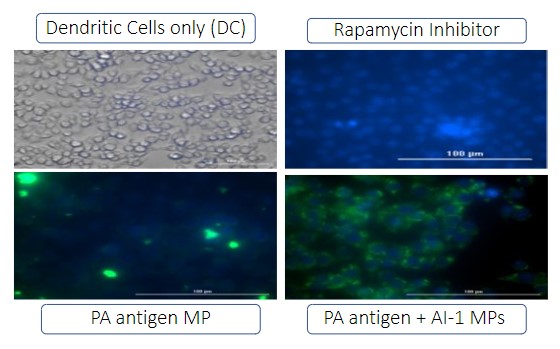
Autophagy is important for antigen cell presentation via major histocompatibility complex I or II. There were four groups in the autophagy study: no treatment group (Dendritic cells only), Inhibitor group (Rapamycin), Antigen group (PA MP antigen), and antigen plus adjuvant group (PA MP antigen + AI-1 MP). Green dye is specifically for autophagosome. PA MP plus AI-1 MP group showed a significantly higher expression of autophagosomes versus the PA antigen MPs.
Formulation and Delivery - Biomolecular - Formulation
Category: Poster Abstract
(W0930-12-70) Novel Sidekick in Vaccine Formulations: Microparticle Autoinducer-1
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- SS
Sarthak Shah, MS
Mercer University
Atlanta, Georgia, United States - SS
Sarthak Shah, MS
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Bacteria can communicate via different signals or inducers. Autoinducer molecules serve as a common communicator or ‘signal’ of both gram positive and negative bacteria. Once this ‘signal’ reaches a threshold, this leads to cumulative dendritic cell activation. This activation allows the human body to initiate an innate immune response, and eventually an adaptive response to the foreign invader. Autoinducer-1 (AI-1) molecule, N-octanoyl-L-Homoserine lactone, serves as a common communicator between both types of bacteria. This molecule is a member of the N-acyl-homoserine lactone family. Previously, DPD or autoinducer-2, was determined immunogenic and had adjuvant potential1. Since the AI-1 compound is a common ‘signal’ for bacterial communication, this compound can be investigated to determine its adjuvant potency in vaccines. Converting this compound into a microparticle allows this compound to appear even more foreign to the body, and thus, mounting a stronger immune response. There are currently only a few FDA approved adjuvants. Not all FDA approved adjuvants work 100% of the time in vaccine formulations of which the primary function is to increase the immune response. Thus, more adjuvants are needed to couple with vaccines that can prevent bacterial and viral infections. We studied the immunological profile of this microparticulate compound and its potential for serving as an adjuvant in vaccine formulations.
Methods: The AI-1 microparticles (MPs) were formulated by a W/O/W double-emulsion solvent evaporation method by using the PLGA (poly (lactic-co-glycolic acid) polymer. This emulsion was then lyophilized. Two antigens, Colonization Factor Antigen I (CFA/I) from E. Coli. and the inactive Protective Antigen (PA) from Bacillus anthracis, were formulated with Bovine Serum Albumin (BSA) polymer. The two antigens were mixed with the crosslinked-albumin matrix and spray dried using Buchi mini spray dryer B-290. Other FDA approved adjuvants: Alum, MF59, and CpG were formulated via spray drying. The MPs were characterized for particle size, charge, and the polydispersity index. The surface morphology was visualized by scanning electron microscopy. In vitro immunogenicity or the innate response was observed via Griess’s assay by measuring the release of nitric oxide (NO) by dendritic cells which is an antigen presenting cell (APC). NO was measured by comparing AI-1 and the FDA approved adjuvants alone and when paired with both antigens (CFA and PA). Autophagy plays an important role in antigen presentation. Hence, the ability of vaccine MP to induce autophagosomes in murine dendritic cells was evaluated using CYTO-ID® Autophagy detection kit. Measurement of encapsulation efficiency and in vitro release of encapsulated protein from microparticles was done via the bicinchoninic acid (BCA) assay. Cytotoxicity was measured via the MTT assay (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide). Adjuvant potential was evaluated by paring adjuvant AI-1 MPs with particulate vaccines for measles, zika virus, influenza, and meningococcal polysaccharide. Griess’s assay analyzed the particulate vaccines with and without combination with different particulate adjuvants as Alum, Addavax (MF59), MPL-A and CpG.
Results: The MP were characterized for particle size, charge, and the polydispersity index: 4.43±0.29 um, -32.0±0.92 mV, and 0.468. The low PDI indicates uniform size distribution of the particles. The negative surface charge indicates the high stability of MP in an aqueous solution. Morphologically, the particles were found to be spherical. Spherical shape indicates better antigen uptake by the Antigen Presenting Cells (APCs). The release of nitric oxide by dendritic cells was comparable to the FDA approved adjuvants. NO release was found to be higher of CFA antigen and AI-1 adjuvant verses the antigen alone. MPs were found to be non-cytotoxic to the dendritic cells. Adjuvant potential was evaluated by paring the adjuvant AI-1 with particulate vaccines. Adjuvanted Vaccine MP (AI-1) showed a comparable NO release versus the FDA approved adjuvants indicating that AI-1 is immunogenic. NO release was significantly higher when AI-1 was paired with particulate vaccines for measles, influenza, and meningococcal polysaccharide, but not for zika virus indicating AI-1 does have adjutancy potential. BCA assay was used to determine encapsulation efficiency and percent release of encapsulated adjuvant from microparticles. Encapsulation efficiency ranged from 51-70%. Approximately 40 % of the encapsulated adjuvant was released within 24 hours indicating that PLGA has an extended-release pattern.
Conclusion: The microparticulate formulations of AI-1 and all other MPs are non-cytotoxic towards the dendritic cells. AI-1 is immunogenic as the FDA approved adjuvants such as Alum, MF59, and CpG. CFA antigen and AI-1 adjuvants yielded better NO release from dendric cells versus PA antigen and AI-1 pair indicating that AI-1 may not work with all antigens. Additional studies with different antigens is needed to have a better understanding of what AI-1 adjuvant works well with. Adjutancy potential was seen when AI-1 was paired with several particulate vaccines indicating that AI-1 can be used for both bacterial and viral infections. More studies are needed with greater number of vaccine candidates and an in vivo study can confirm the adjutancy potential of autoinducer-1.
References: Joshi D, Chbib C, Uddin MN, D'Souza MJ. Evaluation of Microparticulate (S)-4,5-Dihydroxy-2,3-pentanedione (DPD) as a Potential Vaccine Adjuvant. AAPS J. 2021 Jun 15;23(4):84. doi: 10.1208/s12248-021-00617-6. PMID: 34131810.
Acknowledgements: Colonization Factor Antigen I (CFA I) and Protective Antigen (PA) were obtained from BEI resources.

Nitrite oxide release from dendritic cells after stimulation for 24 hrs. Autoinder-1 (AI-1) compared versus the FDA approved adjuvants: Alum, MF59, and CpG. NO release was compared when AI-1 was paired with other adjuvants to observe combination effect. Release of NO was significantly higher when AI-1 was paired with alum, MF59, and CpG MPs.

Release of nitric oxide by dendritic cells was measured when AI-1 MP was paired with two antigens. The first antigen is Colonization Factor Antigen I MP (CFA/I) from E. Coli and the second is Protective Antigen (PA) MP from inactivated Bacillus anthracis. AI-1 MP when paired with CFA MP and PA MP antigen, displayed significantly higher NO release when compared to the blank MPs.

Autophagy is important for antigen cell presentation via major histocompatibility complex I or II. There were four groups in the autophagy study: no treatment group (Dendritic cells only), Inhibitor group (Rapamycin), Antigen group (PA MP antigen), and antigen plus adjuvant group (PA MP antigen + AI-1 MP). Green dye is specifically for autophagosome. PA MP plus AI-1 MP group showed a significantly higher expression of autophagosomes versus the PA antigen MPs.
