Back
Purpose: Immunological rejection is the leading reason for corneal transplantation failure and the overall rejection can occur in 10%~50% of patients 1. Topical corticosteroid eyedrops is the mainstay for both preventing and treating corneal allograft rejection. Due to the poor ocular bioavailability, corticosteroid eyedrops must be applied 4-5 times a day for at least one month, and then gradually taper down the dosing frequency over 6-12 months to prevent corneal graft rejection 2. The requirements of frequent dosing for extended period of time reduces patient adherences which can increase the injection rate. Subconjunctival (SCT) injection of water-soluble dexamethasone sodium phosphate (DSP) delivered high drug level into the eye, unfortunately, free drug solutions quickly cleared from the eye within a day 3. To solve this clinic problem, we aim to develop a long-lasting biodegradable DSP loaded nanoparticle (DSP-NP) that can be administered through SCT injection to provide up to 6 months efficacy for preventing corneal allograft rejection.
Methods: Novel dicarboxyl-terminated PLA-2COOH8.2kDa polymer was designed to formulate water-soluble DSP into nanoparticle with both high drug loading and sustained drug release up to 3 months in vitro (3m DSP-NP) using zinc ion bridge method 4. 3m DSP-NP was fully characterized including particle size, surface charge, morphology, drug loading and in vitro drug release profile. In vivo ocular PK study was conducted after a single SCT injection of 3m DSP-NP (600 µg DSP), DSP and its metabolites dexamethasone (DEX) concentration in rat aqueous humor was quantified using LC/MS/MS. Efficacy study of 3m DSP-NP in preventing corneal allograft rejection was investigated on rat penetrating keratoplasty model (PKP) 4. A single SCT injection of 3m DSP-NP (800 µg DSP) was conducted immediately after the PKP surgery and routine clinical observation (cornea opacity, edema and neovascularization) was performed to exam corneal graft rejection.
Results: Water-soluble DSP was encapsulated into PLA-2COOH8.2kDa NP through zinc ion bridge (Fig A). PLA-2COOH8.2kDa DSP-NP demonstrated spherical shape with particle size around 200 nm (Fig B) and nearly neutral surface charge (-3.6±0.2 mV). PLA-2COOH8.2kDa DSP-NP provided 8 wt% drug loading and sustained in vitro drug release for 3 months (Fig C). 3m DSP-NP (600 µg DSP) provide sustained level of DSP and DEX in rat aqueous humor for 6 months after a single SCT injection (Fig D). Efficacy study demonstrated that the single SCT injection of 3m DSP-NP (800 µg DSP) successfully prevent rat corneal allograft rejection for 6 months as evidenced by clear cornea without edema over 6 months (Fig E). In comparison, untreated graft was rejected at 7 days after the surgery (POD7) as revealed by corneal graft completely lost its transparency with serve edema and neovascularization invading from bed to the graft (Fig F).
Conclusion: These results suggested that the 3m DSP-NP can be a promising approach for sustained delivery of corticosteroids to the eye with improved patient adherence and better outcomes for the management of corneal transplantation immunological rejection.
References: 1. Yin J. Advances in corneal graft rejection. Current Opinion in Ophthalmology. 2021;32(4):331-337.
2. Price FW, Jr., Price DA, Ngakeng V, Price MO. Survey of steroid usage patterns during and after low-risk penetrating keratoplasty. Cornea. 2009;28(8):865-870.
3. Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. American Journal of Ophthalmology. 1999;128(2):192-197.
4. Pan Q, Xu Q, Boylan NJ, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32-40.
Acknowledgements: This study was supported by grants from National Institutes of Health (R01EY027827).
Financial interest: The nanoparticle technology described in this publication is being licensed to GrayBug Vision Inc. Justin Hanes is a founder of GrayBug Vision, Inc. He owns company stock, which is subject to certain rules and restrictions under Johns Hopkins University policy. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict-of-interest policies.
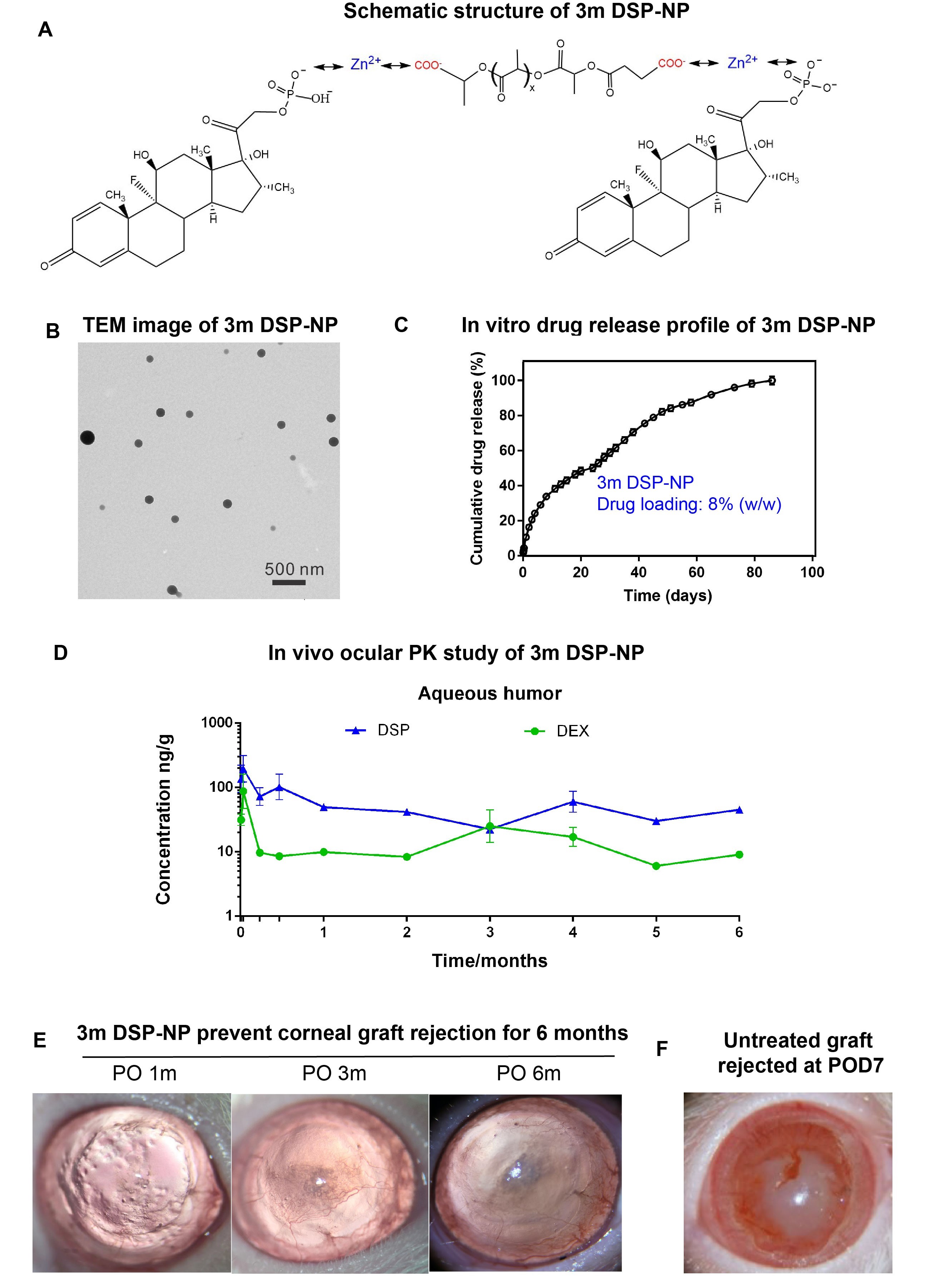
Figure 1: (A) Schematic structure of novel PLA-2COOH DSP-NP. (B) TEM images of 3m DSP-NP. (C) In vitro drug release profiles of 3m DSP-NP under sink conditions at 37°C. (D) A single SCT injection of 3m DSP-NP (600 µg DSP) provided sustained DSP and DEX release in rat aqueous humor. (E) A single SCT injection of 3m DSP-NP (800 µg DSP) prevented corneal graft rejection for 6 months. Representative images of grafts received 3m DSP-NP treatment at 1m, 3m and 6m after PKP surgery. (F) Untreated graft was rejected at POD7.
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(W0930-12-69) A Single Dose of Nanoparticle Provides 6 Months Efficacy for Preventing Rat Corneal Allograft Rejection
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET

Tuo Meng, PhD
Senior scientist
Merck
RICHMOND, Virginia, United States
Tuo Meng, PhD
Senior scientist
Merck
RICHMOND, Virginia, United States
Presenting Author(s)
Main Author(s)
Purpose: Immunological rejection is the leading reason for corneal transplantation failure and the overall rejection can occur in 10%~50% of patients 1. Topical corticosteroid eyedrops is the mainstay for both preventing and treating corneal allograft rejection. Due to the poor ocular bioavailability, corticosteroid eyedrops must be applied 4-5 times a day for at least one month, and then gradually taper down the dosing frequency over 6-12 months to prevent corneal graft rejection 2. The requirements of frequent dosing for extended period of time reduces patient adherences which can increase the injection rate. Subconjunctival (SCT) injection of water-soluble dexamethasone sodium phosphate (DSP) delivered high drug level into the eye, unfortunately, free drug solutions quickly cleared from the eye within a day 3. To solve this clinic problem, we aim to develop a long-lasting biodegradable DSP loaded nanoparticle (DSP-NP) that can be administered through SCT injection to provide up to 6 months efficacy for preventing corneal allograft rejection.
Methods: Novel dicarboxyl-terminated PLA-2COOH8.2kDa polymer was designed to formulate water-soluble DSP into nanoparticle with both high drug loading and sustained drug release up to 3 months in vitro (3m DSP-NP) using zinc ion bridge method 4. 3m DSP-NP was fully characterized including particle size, surface charge, morphology, drug loading and in vitro drug release profile. In vivo ocular PK study was conducted after a single SCT injection of 3m DSP-NP (600 µg DSP), DSP and its metabolites dexamethasone (DEX) concentration in rat aqueous humor was quantified using LC/MS/MS. Efficacy study of 3m DSP-NP in preventing corneal allograft rejection was investigated on rat penetrating keratoplasty model (PKP) 4. A single SCT injection of 3m DSP-NP (800 µg DSP) was conducted immediately after the PKP surgery and routine clinical observation (cornea opacity, edema and neovascularization) was performed to exam corneal graft rejection.
Results: Water-soluble DSP was encapsulated into PLA-2COOH8.2kDa NP through zinc ion bridge (Fig A). PLA-2COOH8.2kDa DSP-NP demonstrated spherical shape with particle size around 200 nm (Fig B) and nearly neutral surface charge (-3.6±0.2 mV). PLA-2COOH8.2kDa DSP-NP provided 8 wt% drug loading and sustained in vitro drug release for 3 months (Fig C). 3m DSP-NP (600 µg DSP) provide sustained level of DSP and DEX in rat aqueous humor for 6 months after a single SCT injection (Fig D). Efficacy study demonstrated that the single SCT injection of 3m DSP-NP (800 µg DSP) successfully prevent rat corneal allograft rejection for 6 months as evidenced by clear cornea without edema over 6 months (Fig E). In comparison, untreated graft was rejected at 7 days after the surgery (POD7) as revealed by corneal graft completely lost its transparency with serve edema and neovascularization invading from bed to the graft (Fig F).
Conclusion: These results suggested that the 3m DSP-NP can be a promising approach for sustained delivery of corticosteroids to the eye with improved patient adherence and better outcomes for the management of corneal transplantation immunological rejection.
References: 1. Yin J. Advances in corneal graft rejection. Current Opinion in Ophthalmology. 2021;32(4):331-337.
2. Price FW, Jr., Price DA, Ngakeng V, Price MO. Survey of steroid usage patterns during and after low-risk penetrating keratoplasty. Cornea. 2009;28(8):865-870.
3. Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. American Journal of Ophthalmology. 1999;128(2):192-197.
4. Pan Q, Xu Q, Boylan NJ, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32-40.
Acknowledgements: This study was supported by grants from National Institutes of Health (R01EY027827).
Financial interest: The nanoparticle technology described in this publication is being licensed to GrayBug Vision Inc. Justin Hanes is a founder of GrayBug Vision, Inc. He owns company stock, which is subject to certain rules and restrictions under Johns Hopkins University policy. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict-of-interest policies.

Figure 1: (A) Schematic structure of novel PLA-2COOH DSP-NP. (B) TEM images of 3m DSP-NP. (C) In vitro drug release profiles of 3m DSP-NP under sink conditions at 37°C. (D) A single SCT injection of 3m DSP-NP (600 µg DSP) provided sustained DSP and DEX release in rat aqueous humor. (E) A single SCT injection of 3m DSP-NP (800 µg DSP) prevented corneal graft rejection for 6 months. Representative images of grafts received 3m DSP-NP treatment at 1m, 3m and 6m after PKP surgery. (F) Untreated graft was rejected at POD7.
