Back
Purpose: Fluid viscosity is much higher in the fed than in the fasted state (Radwan et al., 2014). However, even the viscosity of the fasted human gastric fluid has been measured to be higher than that of water or simulated intestinal fluids due to the presence of protein, mucus and lipids, and a range of 1.7-9.3 mPa.s has been reported (Pedersen et al., 2013). Viscosity can affect drug dissolution rate as it can reduce drug diffusion according to the Stokes-Einstein equation. In addition, increased viscosity will increase drag and therefore reduce particle relative velocity, ultimately reducing dissolution rate. However, it could also potentially increase dissolution rate through an improved lifting and dispersal of drug particles (D’Arcy and Persoons, 2019). This study investigated the effects of increasing medium viscosity through two viscosity enhancing agents (VEAs) (hydroxypropylmethylcellulose (HPMC) and sucrose) on the in vitro dissolution of a BCS class II drug (ibuprofen) in the USP 4 flow-through apparatus (FTA). Each VEA has the potential to affect the dissolution rate differently (Sarisuta and Parrott, 1982) due to their effects on density, solubility and different impacts on molecular diffusion. High solubility (pH 6.8 phosphate buffer) and low solubility (0.1M HCl) media were used and two fluid velocity profiles were attained. Three viscosity values were investigated, namely 0.7 mPa.s, the viscosity of water and aqueous buffers at 37oC; 1.4 mPa.s, approximately the viscosity of milk (Klein et al., 2004), which has been used to simulate gastric fed state (Baxevanis et al., 2016); and 4.5-5.5 mPa.s, the mid-range of observed viscosity of the human gastric fluid in the fasted state (Pedersen et al., 2013).
Methods: Dissolution tests were conducted in the FTA (CE7, closed loop, Sotax AG) with 5 mg of ibuprofen powder (Glentham Life Sciences Ltd) in pH 6.8 phosphate buffer – sampling 2 mL at 2, 4, 8, 15, 30, 45, 60, 90 and 120 minutes - and 0.1M HCl (Honeywell Fluka®) – sampling 2 mL at 10, 20, 40, 60, 90, 150, 210 and 270 minutes, with 0.003% w/v Tween (Sigma-Aldrich), at 8 ml/min (22.6 mm cell) (low velocity) and 16 ml/min (12 mm cell) (high velocity), either (a) without any viscosity enhancing agents (b) with 25% w/v sucrose (phosphate buffer and 0.1M HCl) or 0.3% w/v HPMC (viscosity of 1.4 mPa.s) and (c) with 59% w/v sucrose (phosphate buffer) or 1.05% w/v HPMC (viscosity of 4.4-5.5 mPas). Viscosity was measured at 37oC using an AND Vibro viscometer SV-10. The apparatus was calibrated with deionized water at 37oC. Solubility tests were carried out by adding an excess amount of drug to the relevant media and placing the solutions in a shaking water bath at 100 rpm and 37oC sampling at 1, 5 and 24. All samples were analysed by UV-Vis spectrophotometry (PharmaSpec 1700, Shimadzu) at 222 nm. Density tests were carried out using a density bottle through comparison (n=3) of the weight of each medium at 37oC with water at 37oC as a reference weight and density.
Results: In 0.1M HCl, increasing sucrose concentration resulted in a decrease in the dissolution rate in both fluid velocity environments (Figure 1). This could be due to the reduction of drug diffusivity by the establishment of hydrogen bonds between sucrose and water (Nelson and Shah, 1987; Shah and Nelson, 1987). In phosphate buffer, the reduction in dissolution was only clear in the low velocity environment when the viscosity was increased to 4.5 mPa.s. HPMC had a lesser effect than sucrose, as a reduction in dissolution rate with increased viscosity was only observed in the faster velocity environment in 0.1M HCl (Figure 2). This could be due to the amphiphilicity and wetting capacity of HPMC which can increase the surface area available for dissolution and hence the dissolution rate (Javeer and Amin, 2014; Tundisi et al., 2021). The fluid density and related effects on particle motion, but not solubility, may have impacted the observed results from each VEA, as none of the solubility values of media containing VEA were statically significantly different from media without VEA at 24 hours (t-test, α = 0.05). However, sucrose had a greater impact on density than HPMC at the concentrations used (Table 1). The effect of fluid velocity in increasing dissolution was reduced with the increased viscosity induced by both VEAs in 0.1M HCl. In phosphate buffer, due to the fast nature of the dissolution, the interplay between flow rate/fluid velocity and viscosity needs investigating.
Conclusion: Moderate (≤ 5.5 mPa.s) increases in viscosity over that of water at 37oC were observed to affect dissolution of ibuprofen particles, in particular in low solubility medium. The VEA used and fluid velocity environment can impact the observed result, and the effect of particle motion vs diffusion in different viscosity environments warrants further investigation.
References: 1. Radwan, A., Wagner, M., Amidon, G. L., Langguth, P. Bio-predictive tablet disintegration: Effect of water diffusivity, fluid flow, food composition and test conditions. European Journal of Pharmaceutical Sciences. 57, 273-279 (2014).
2. Pedersen, P. B., Vilmann, P., Bar-Shalom, D., Müllertz, A. Characterization of fasted human gastric fluid for relevant rheological parameters and gastric lipase activities. European Journal of Pharmaceutics and Biopharmaceutics. 85, 958-965 (2013).
3. D'Arcy, D. M., Persoons, T. Understanding the Potential for Dissolution Simulation to Explore the Effects of Medium Viscosity on Particulate Dissolution. American Association of Pharmaceutical Science. 20, 1–13 (2019).
4. Klein, S., Butler, J., Hempenstall, J.M., Reppas, C., Dressman, J.B. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. Journal of Pharmacy and Pharmacology. 56, 605-610 (2004).
5. Baxevanis, F., Kuiper, J., Fotaki, N. Fed-state gastric media and drug analysis techniques: Current status and points to consider. European Journal of Pharmaceutics and Biopharmaceutics. 107, 234-248 (2016).
6. Sarisuta, N., Parrott, E. L. Relationship of Dissolution Rate to Viscosity of Polymeric Solutions. Journal of Pharmaceutical Sciences. 71, 1375-1380 (1982).
7. Nelson, K.G., Shah, A.C. Mass transport in dissolution kinetics. I: Convective diffusion to assess the role of fluid viscosity under forced flow conditions. Journal of Pharmaceutical Sciences. 76, 799-802 (1987).
8. Shah, A.C., Nelson, K.G. Mass transport in dissolution kinetics. II: Convective diffusion to assess role of viscosity under conditions of gravitational flow. Journal of Pharmaceutical Sciences. 76, 910-913 (1987).
9. Shi, N.Q., Jin, Y., Zhang, Y., Che, X.X., Xiao, X., Cui, G.H., Chen, Y.Z., Feng, B., Li, Z.Q., Qi, X.R. The Influence of Cellulosic Polymer's Variables on Dissolution/Solubility of Amorphous Felodipine and Crystallization Inhibition from a Supersaturated State. AAPS PharmSciTech. 20, 12 (2018).
10. Javeer, S. D., Amin, P. D. Solubility and dissolution enhancement of HPMC-based solid dispersions of carbamazepine by hot-melt extrusion technique. Asian Journal of Pharmaceutics. 8, 119-124 (2014).
Acknowledgements:
Funding: Science Foundation Ireland, co-funded under the European Regional Development Fund (Grant number 12/RC/2275_P2)
.jpg)
Figure 1. Dissolution profiles of 5 mg of 160 µm median diameter ibuprofen particles in 200 ml of pH 6.8 phosphate buffer (filled icons) or 0.1M HCl (empty icons) with no sucrose (yellow), 25% w/v sucrose (red), or 59% w/v sucrose (blue), with 0.003% w/v Tween 20 at 37oC in the FTA at two average linear fluid velocities: 0.33 mm/s (squares) and 2.35 mm/s (circles).
.jpg)
Figure 2. Dissolution profiles of 5 mg of 160 µm median diameter ibuprofen particles in 200 ml of pH 6.8 phosphate buffer (filled icons) or 0.1M HCl (empty icons) with no HPMC (yellow), 0.3% w/v HPMC (red), or 1.05% w/v HPMC (blue), with 0.003% w/v Tween 20 at 37oC in the FTA at two average linear fluid velocities: 0.33 mm/s (squares) and 2.35 mm/s (circles).
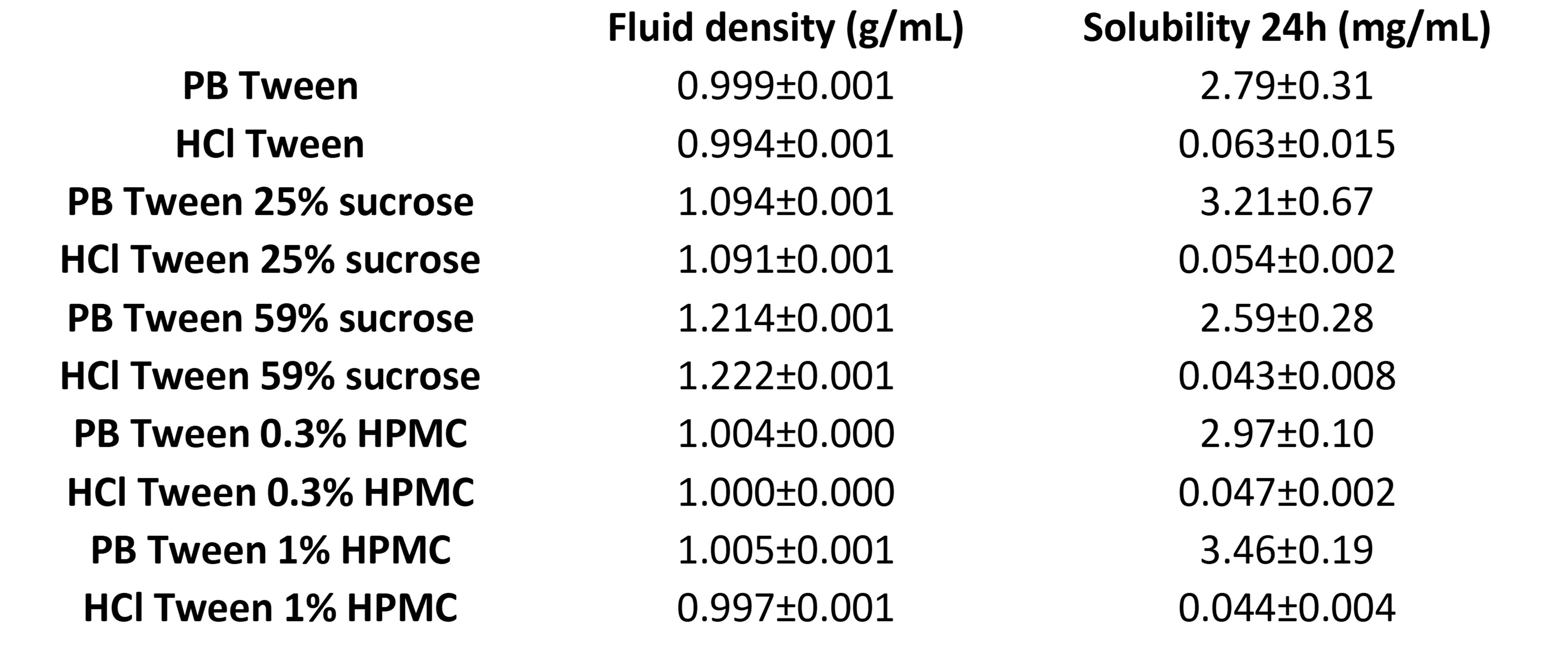
Table 1. Fluid density and ibuprofen saturation solubility after 24h of incubation at 37oC and 100 rpm in different media used for dissolution testing.
Manufacturing and Analytical Characterization - Chemical - Analytical
Category: Poster Abstract
(W0930-08-44) Effect of Moderate Biorelevant Increases in Medium Viscosity on the Dissolution Rate of Ibuprofen Particles
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- MN
Marina Navas-Bachiller, BS
Trinity College Dublin
Dublin, Dublin, Ireland - MN
Marina Navas-Bachiller, BS
Trinity College Dublin
Dublin, Dublin, Ireland
Presenting Author(s)
Main Author(s)
Purpose: Fluid viscosity is much higher in the fed than in the fasted state (Radwan et al., 2014). However, even the viscosity of the fasted human gastric fluid has been measured to be higher than that of water or simulated intestinal fluids due to the presence of protein, mucus and lipids, and a range of 1.7-9.3 mPa.s has been reported (Pedersen et al., 2013). Viscosity can affect drug dissolution rate as it can reduce drug diffusion according to the Stokes-Einstein equation. In addition, increased viscosity will increase drag and therefore reduce particle relative velocity, ultimately reducing dissolution rate. However, it could also potentially increase dissolution rate through an improved lifting and dispersal of drug particles (D’Arcy and Persoons, 2019). This study investigated the effects of increasing medium viscosity through two viscosity enhancing agents (VEAs) (hydroxypropylmethylcellulose (HPMC) and sucrose) on the in vitro dissolution of a BCS class II drug (ibuprofen) in the USP 4 flow-through apparatus (FTA). Each VEA has the potential to affect the dissolution rate differently (Sarisuta and Parrott, 1982) due to their effects on density, solubility and different impacts on molecular diffusion. High solubility (pH 6.8 phosphate buffer) and low solubility (0.1M HCl) media were used and two fluid velocity profiles were attained. Three viscosity values were investigated, namely 0.7 mPa.s, the viscosity of water and aqueous buffers at 37oC; 1.4 mPa.s, approximately the viscosity of milk (Klein et al., 2004), which has been used to simulate gastric fed state (Baxevanis et al., 2016); and 4.5-5.5 mPa.s, the mid-range of observed viscosity of the human gastric fluid in the fasted state (Pedersen et al., 2013).
Methods: Dissolution tests were conducted in the FTA (CE7, closed loop, Sotax AG) with 5 mg of ibuprofen powder (Glentham Life Sciences Ltd) in pH 6.8 phosphate buffer – sampling 2 mL at 2, 4, 8, 15, 30, 45, 60, 90 and 120 minutes - and 0.1M HCl (Honeywell Fluka®) – sampling 2 mL at 10, 20, 40, 60, 90, 150, 210 and 270 minutes, with 0.003% w/v Tween (Sigma-Aldrich), at 8 ml/min (22.6 mm cell) (low velocity) and 16 ml/min (12 mm cell) (high velocity), either (a) without any viscosity enhancing agents (b) with 25% w/v sucrose (phosphate buffer and 0.1M HCl) or 0.3% w/v HPMC (viscosity of 1.4 mPa.s) and (c) with 59% w/v sucrose (phosphate buffer) or 1.05% w/v HPMC (viscosity of 4.4-5.5 mPas). Viscosity was measured at 37oC using an AND Vibro viscometer SV-10. The apparatus was calibrated with deionized water at 37oC. Solubility tests were carried out by adding an excess amount of drug to the relevant media and placing the solutions in a shaking water bath at 100 rpm and 37oC sampling at 1, 5 and 24. All samples were analysed by UV-Vis spectrophotometry (PharmaSpec 1700, Shimadzu) at 222 nm. Density tests were carried out using a density bottle through comparison (n=3) of the weight of each medium at 37oC with water at 37oC as a reference weight and density.
Results: In 0.1M HCl, increasing sucrose concentration resulted in a decrease in the dissolution rate in both fluid velocity environments (Figure 1). This could be due to the reduction of drug diffusivity by the establishment of hydrogen bonds between sucrose and water (Nelson and Shah, 1987; Shah and Nelson, 1987). In phosphate buffer, the reduction in dissolution was only clear in the low velocity environment when the viscosity was increased to 4.5 mPa.s. HPMC had a lesser effect than sucrose, as a reduction in dissolution rate with increased viscosity was only observed in the faster velocity environment in 0.1M HCl (Figure 2). This could be due to the amphiphilicity and wetting capacity of HPMC which can increase the surface area available for dissolution and hence the dissolution rate (Javeer and Amin, 2014; Tundisi et al., 2021). The fluid density and related effects on particle motion, but not solubility, may have impacted the observed results from each VEA, as none of the solubility values of media containing VEA were statically significantly different from media without VEA at 24 hours (t-test, α = 0.05). However, sucrose had a greater impact on density than HPMC at the concentrations used (Table 1). The effect of fluid velocity in increasing dissolution was reduced with the increased viscosity induced by both VEAs in 0.1M HCl. In phosphate buffer, due to the fast nature of the dissolution, the interplay between flow rate/fluid velocity and viscosity needs investigating.
Conclusion: Moderate (≤ 5.5 mPa.s) increases in viscosity over that of water at 37oC were observed to affect dissolution of ibuprofen particles, in particular in low solubility medium. The VEA used and fluid velocity environment can impact the observed result, and the effect of particle motion vs diffusion in different viscosity environments warrants further investigation.
References: 1. Radwan, A., Wagner, M., Amidon, G. L., Langguth, P. Bio-predictive tablet disintegration: Effect of water diffusivity, fluid flow, food composition and test conditions. European Journal of Pharmaceutical Sciences. 57, 273-279 (2014).
2. Pedersen, P. B., Vilmann, P., Bar-Shalom, D., Müllertz, A. Characterization of fasted human gastric fluid for relevant rheological parameters and gastric lipase activities. European Journal of Pharmaceutics and Biopharmaceutics. 85, 958-965 (2013).
3. D'Arcy, D. M., Persoons, T. Understanding the Potential for Dissolution Simulation to Explore the Effects of Medium Viscosity on Particulate Dissolution. American Association of Pharmaceutical Science. 20, 1–13 (2019).
4. Klein, S., Butler, J., Hempenstall, J.M., Reppas, C., Dressman, J.B. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. Journal of Pharmacy and Pharmacology. 56, 605-610 (2004).
5. Baxevanis, F., Kuiper, J., Fotaki, N. Fed-state gastric media and drug analysis techniques: Current status and points to consider. European Journal of Pharmaceutics and Biopharmaceutics. 107, 234-248 (2016).
6. Sarisuta, N., Parrott, E. L. Relationship of Dissolution Rate to Viscosity of Polymeric Solutions. Journal of Pharmaceutical Sciences. 71, 1375-1380 (1982).
7. Nelson, K.G., Shah, A.C. Mass transport in dissolution kinetics. I: Convective diffusion to assess the role of fluid viscosity under forced flow conditions. Journal of Pharmaceutical Sciences. 76, 799-802 (1987).
8. Shah, A.C., Nelson, K.G. Mass transport in dissolution kinetics. II: Convective diffusion to assess role of viscosity under conditions of gravitational flow. Journal of Pharmaceutical Sciences. 76, 910-913 (1987).
9. Shi, N.Q., Jin, Y., Zhang, Y., Che, X.X., Xiao, X., Cui, G.H., Chen, Y.Z., Feng, B., Li, Z.Q., Qi, X.R. The Influence of Cellulosic Polymer's Variables on Dissolution/Solubility of Amorphous Felodipine and Crystallization Inhibition from a Supersaturated State. AAPS PharmSciTech. 20, 12 (2018).
10. Javeer, S. D., Amin, P. D. Solubility and dissolution enhancement of HPMC-based solid dispersions of carbamazepine by hot-melt extrusion technique. Asian Journal of Pharmaceutics. 8, 119-124 (2014).
Acknowledgements:
Funding: Science Foundation Ireland, co-funded under the European Regional Development Fund (Grant number 12/RC/2275_P2)
.jpg)
Figure 1. Dissolution profiles of 5 mg of 160 µm median diameter ibuprofen particles in 200 ml of pH 6.8 phosphate buffer (filled icons) or 0.1M HCl (empty icons) with no sucrose (yellow), 25% w/v sucrose (red), or 59% w/v sucrose (blue), with 0.003% w/v Tween 20 at 37oC in the FTA at two average linear fluid velocities: 0.33 mm/s (squares) and 2.35 mm/s (circles).
.jpg)
Figure 2. Dissolution profiles of 5 mg of 160 µm median diameter ibuprofen particles in 200 ml of pH 6.8 phosphate buffer (filled icons) or 0.1M HCl (empty icons) with no HPMC (yellow), 0.3% w/v HPMC (red), or 1.05% w/v HPMC (blue), with 0.003% w/v Tween 20 at 37oC in the FTA at two average linear fluid velocities: 0.33 mm/s (squares) and 2.35 mm/s (circles).

Table 1. Fluid density and ibuprofen saturation solubility after 24h of incubation at 37oC and 100 rpm in different media used for dissolution testing.
