Back
Purpose: There has been a rapid growth in the investigation of Adeno-associated virus (AAV)-based gene therapeutics in the past decades. Viral vectors packaged with genetic material are delivered to targeted cells in human patients for the expression of therapeutic proteins. However, pre-existing and treatment-induced/boosted neutralizing antibodies can significantly impair viral transduction and, by extension, impact drug efficacy. This work focused on the de novo development and validation of a flow cytometry (FC) assay for the detection of neutralizing antibodies (NAbs) against AAV5.
Methods: During initial feasibility stage, multiple platforms and vendors were evaluated to select a system that can provide good potency of viral transduction into the cell line. Fluorescence readout using a plate reader was tested first, but resulted in high background with no distinct potency curve. Therefore a flow cytometry approach was considered. Two models of flow cytometers, a Novocyte Quanteon (model 1) and a FACS Canto II (model 2) were compared. The Quanteon (model 1) showed superior sensitivity and signal-to-noise (S/N) > 20, compared to Canto (model 2), which has a S/N of 2 (Figure 1). In addition, a clear separation of negative and positive population can be seen from the Quanteon plot, which allows for %GFP+ gating (Figure 3a). Compared to traditional MFI (mean or median), %GFP output provides better reproducibility and robustness for this assay. The principle of the fully developed FC method is as follows: continuously cultured cells are plated on the day of assay. Samples and controls are incubated with AAV5-GFP for antibody-drug interaction. After that, the sample/virus mixture is loaded onto the cell plate and incubate for 2 days. After incubation, the cells are harvested and acquired on the flow cytometer for the measurement of GFP expression.
Results: The FC Nab assay was successfully validated with optimal results. Due to the incidences of pre-existing antibodies in human serum, negative base pool and cut point lots were pre-screened to have an antibody naïve population. Parametric assay cut point was derived from data points generated from six runs by two analysts. Subsequently, sensitivity was determined from eight curves generated by two analysts. An average cut point interpolation at 61 ng/mL of PC antibody and an assay sensitivity of 90 ng/mL was achieved with great titer precision (Figure 2). Precision runs were carried out by two analysts over six plates, and inter- and intra-assay precision for all levels of controls were within acceptability (25% CV) parameters for both raw %GFP and %Neutralization (Figure 3b). Evaluations of drug tolerance, selectivity, stability and robustness all met acceptance criteria.
Conclusion: Flow cytometry platform combined with %GFP output greatly improved dynamic range and sensitivity of the neutralizing antibody assay for AAV5. The method was successfully validated, and is in active use for analysis of clinical samples. A similar approach can be employed to develop and validate Nab methods against other serotypes of AAV or for other types of gene therapy products utilizing viral vectors.
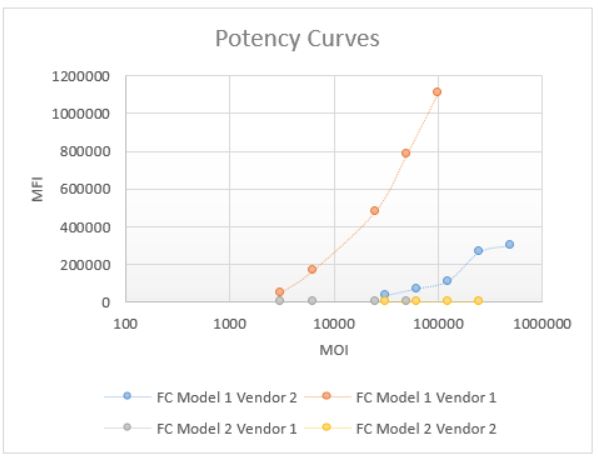
Comparison of Potency Curves Read on Different Instrument using Different Vendors of AAVs
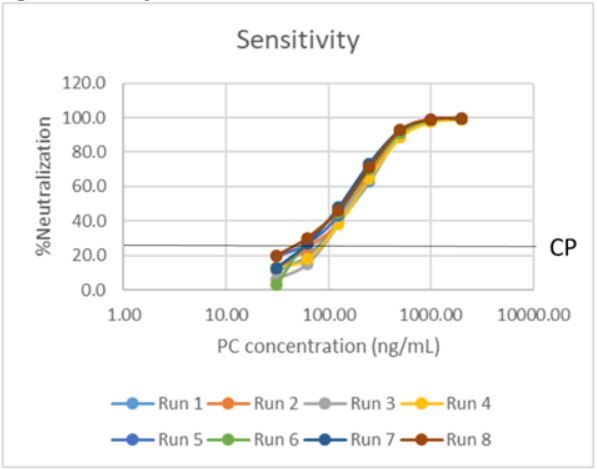
Sensitivity Curves
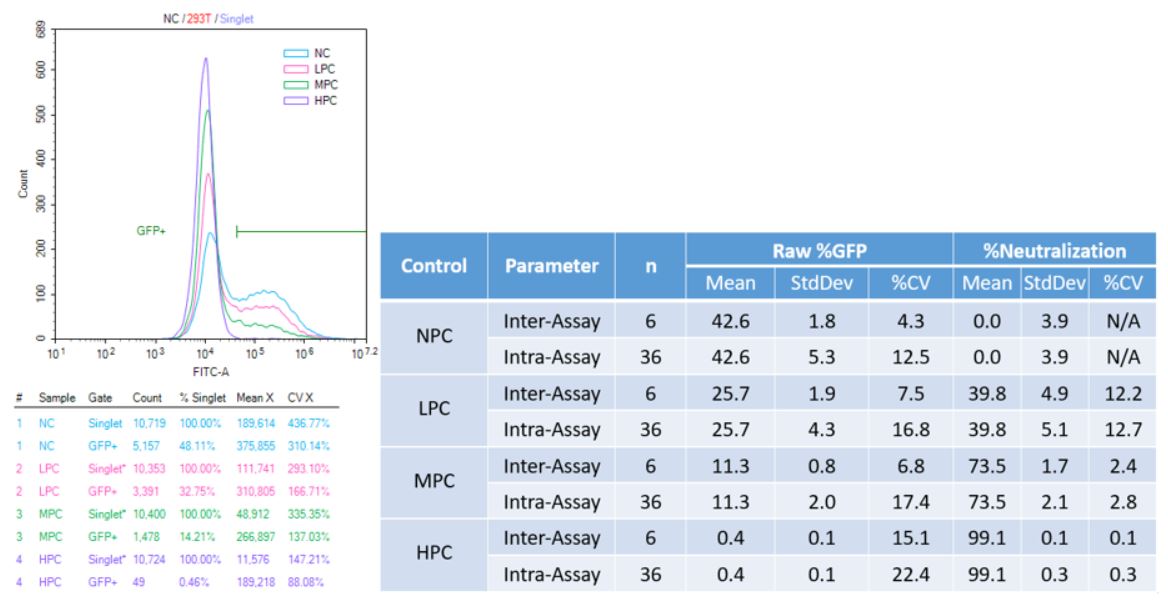
Figure 3 Validation Precision
a) Representative overlay plots and statistics of controls
b) Precision Result Summary Table
Bioanalytics - Biomolecular - Immunogenicity
Category: Poster Abstract
(M1430-09-50) De Novo Development and Validation of a Flow Cytometry Method for the Detection of Neutralizing Antibodies against Adeno-Associated Virus Serotype 5 (AAV5)
Monday, October 17, 2022
2:30 PM – 3:30 PM ET
- RW
Ruqi Wang, Ph.D.
Eurofins
Saint Charles, Missouri, United States - RW
Ruqi Wang, Ph.D.
Eurofins
Saint Charles, Missouri, United States
Presenting Author(s)
Main Author(s)
Purpose: There has been a rapid growth in the investigation of Adeno-associated virus (AAV)-based gene therapeutics in the past decades. Viral vectors packaged with genetic material are delivered to targeted cells in human patients for the expression of therapeutic proteins. However, pre-existing and treatment-induced/boosted neutralizing antibodies can significantly impair viral transduction and, by extension, impact drug efficacy. This work focused on the de novo development and validation of a flow cytometry (FC) assay for the detection of neutralizing antibodies (NAbs) against AAV5.
Methods: During initial feasibility stage, multiple platforms and vendors were evaluated to select a system that can provide good potency of viral transduction into the cell line. Fluorescence readout using a plate reader was tested first, but resulted in high background with no distinct potency curve. Therefore a flow cytometry approach was considered. Two models of flow cytometers, a Novocyte Quanteon (model 1) and a FACS Canto II (model 2) were compared. The Quanteon (model 1) showed superior sensitivity and signal-to-noise (S/N) > 20, compared to Canto (model 2), which has a S/N of 2 (Figure 1). In addition, a clear separation of negative and positive population can be seen from the Quanteon plot, which allows for %GFP+ gating (Figure 3a). Compared to traditional MFI (mean or median), %GFP output provides better reproducibility and robustness for this assay. The principle of the fully developed FC method is as follows: continuously cultured cells are plated on the day of assay. Samples and controls are incubated with AAV5-GFP for antibody-drug interaction. After that, the sample/virus mixture is loaded onto the cell plate and incubate for 2 days. After incubation, the cells are harvested and acquired on the flow cytometer for the measurement of GFP expression.
Results: The FC Nab assay was successfully validated with optimal results. Due to the incidences of pre-existing antibodies in human serum, negative base pool and cut point lots were pre-screened to have an antibody naïve population. Parametric assay cut point was derived from data points generated from six runs by two analysts. Subsequently, sensitivity was determined from eight curves generated by two analysts. An average cut point interpolation at 61 ng/mL of PC antibody and an assay sensitivity of 90 ng/mL was achieved with great titer precision (Figure 2). Precision runs were carried out by two analysts over six plates, and inter- and intra-assay precision for all levels of controls were within acceptability (25% CV) parameters for both raw %GFP and %Neutralization (Figure 3b). Evaluations of drug tolerance, selectivity, stability and robustness all met acceptance criteria.
Conclusion: Flow cytometry platform combined with %GFP output greatly improved dynamic range and sensitivity of the neutralizing antibody assay for AAV5. The method was successfully validated, and is in active use for analysis of clinical samples. A similar approach can be employed to develop and validate Nab methods against other serotypes of AAV or for other types of gene therapy products utilizing viral vectors.

Comparison of Potency Curves Read on Different Instrument using Different Vendors of AAVs

Sensitivity Curves

Figure 3 Validation Precision
a) Representative overlay plots and statistics of controls
b) Precision Result Summary Table
