Back
Purpose: pH-elevated drug-drug interaction (DDI) may impact drug product’s exposure when co-administration with acid-reducing agents (ARAs), which can increase gastric pH from 1-2 to 4-7. It is critical to identify the ARA-DDI early in drug development to guide formulation selection, clinical study design, and labeling restriction. To be noted, ARA-DDI is compound, formulation, and prandial status dependent. PBBM is a powerful tool to describe or predict a drug product’s exposure over time by incorporating physiological, compound-specific properties, and relevant factors into the simulation. For ARA-DDI risk assessment, PBBM can capture the changes in physiology (e.g. gastric pH, gastric transit time), metabolism pathways, and dissolution rate to predict drug exposure in preclinical and clinical so to aid formulation development and clinical design [1].
Methods: Compound A is a weak base compound with highly pH-dependent solubility and a fast precipitation rate. The free base and amorphous form formulations were developed and evaluated in in vitro dissolution testing with biorelevant media and in vivo dog PK studies for ARA-DDI assessment. PBBM was applied to describe pentagastrin and famotidine pretreated dog PK and extrapolated to human for risk assessment.
Results: PBBM identified a significantly reduced exposure for the free base formulation at normal and elevated gastric pH compared to the amorphous formulation. Little or no pH effects on the PK with the amorphous formulation were predicted. This indicates a low risk of ARA-DDI for amorphous formulation compared to free base at projected human dose. A two stage dissolution in SGF/acetate buffer – FaSSIF indicated a fast precipitation for the free form, while amorphous form maintained supersaturation in the second stage in both cases. The 2-step dissolution method was biorelevant and biopredictive and was consistent with in vivo PK data. In the dog PK study, the free base formulation had low and pH dependent PK, as the F was 86% lower in untreated dogs compared to those treated with pentagastrin. For the amorphous formulation PK was high and overall pH-independent, as comparable %F was observed in pentagastrin and famotidine treated dogs at 2 and 5mpk. This observation is consistent with in vitro, in vivo, and in silico evaluations, which indicates amorphous formulation has maintained supersaturation in the small intestine compartments.
Conclusion: In conclusion, PBBM has successfully identified the amorphous formulation could potentially overcome ARA-DDI, which was confirmed by biopredictive in vitro dissolution and in vivo dog studies.
References: [1] Wen L. et al. Applications, challenges, and outlook for PBPK modeling and simulation: A regulatory, industrial and academic perspective. Pharm Res. Approved.
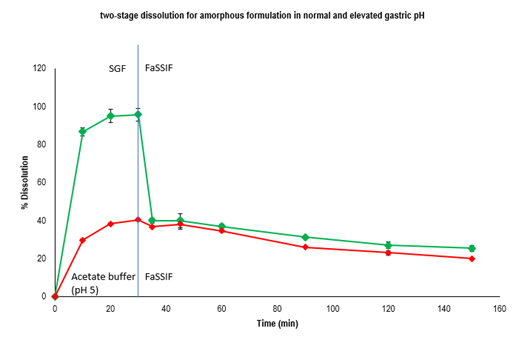
Figure 1. two-stage dissolution in SGF/ARA condition and FaSSIF media for amorphous formulation
Preclinical Development - Chemical - Translation
Category: Poster Abstract
(M1330-02-10) Physiological Based Biopharmaceutics Modeling (PBBM) for pH-dependent DDI Risk Identification and Formulation Selection for a Weak Base Compound
Monday, October 17, 2022
1:30 PM – 2:30 PM ET
- DW
Di Wu, PhD
Sr Scientist
Merck & Co
Rahway, New Jersey, United States - DW
Di Wu, PhD
Sr Scientist
Merck & Co
Rahway, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: pH-elevated drug-drug interaction (DDI) may impact drug product’s exposure when co-administration with acid-reducing agents (ARAs), which can increase gastric pH from 1-2 to 4-7. It is critical to identify the ARA-DDI early in drug development to guide formulation selection, clinical study design, and labeling restriction. To be noted, ARA-DDI is compound, formulation, and prandial status dependent. PBBM is a powerful tool to describe or predict a drug product’s exposure over time by incorporating physiological, compound-specific properties, and relevant factors into the simulation. For ARA-DDI risk assessment, PBBM can capture the changes in physiology (e.g. gastric pH, gastric transit time), metabolism pathways, and dissolution rate to predict drug exposure in preclinical and clinical so to aid formulation development and clinical design [1].
Methods: Compound A is a weak base compound with highly pH-dependent solubility and a fast precipitation rate. The free base and amorphous form formulations were developed and evaluated in in vitro dissolution testing with biorelevant media and in vivo dog PK studies for ARA-DDI assessment. PBBM was applied to describe pentagastrin and famotidine pretreated dog PK and extrapolated to human for risk assessment.
Results: PBBM identified a significantly reduced exposure for the free base formulation at normal and elevated gastric pH compared to the amorphous formulation. Little or no pH effects on the PK with the amorphous formulation were predicted. This indicates a low risk of ARA-DDI for amorphous formulation compared to free base at projected human dose. A two stage dissolution in SGF/acetate buffer – FaSSIF indicated a fast precipitation for the free form, while amorphous form maintained supersaturation in the second stage in both cases. The 2-step dissolution method was biorelevant and biopredictive and was consistent with in vivo PK data. In the dog PK study, the free base formulation had low and pH dependent PK, as the F was 86% lower in untreated dogs compared to those treated with pentagastrin. For the amorphous formulation PK was high and overall pH-independent, as comparable %F was observed in pentagastrin and famotidine treated dogs at 2 and 5mpk. This observation is consistent with in vitro, in vivo, and in silico evaluations, which indicates amorphous formulation has maintained supersaturation in the small intestine compartments.
Conclusion: In conclusion, PBBM has successfully identified the amorphous formulation could potentially overcome ARA-DDI, which was confirmed by biopredictive in vitro dissolution and in vivo dog studies.
References: [1] Wen L. et al. Applications, challenges, and outlook for PBPK modeling and simulation: A regulatory, industrial and academic perspective. Pharm Res. Approved.

Figure 1. two-stage dissolution in SGF/ARA condition and FaSSIF media for amorphous formulation
