Back
Purpose: Selective laser sintering (SLS) is a single-step three-dimensional printing (3DP) process that is gaining momentum in pharmaceutical dosage form manufacturing. This novel 3DP process offers opportunities for manufacturing various pharmaceutical dosage forms with a wide array of drug delivery systems. The purpose of this research was to introduce carbonyl iron as a multi-functional magnetic and heat conductive ingredient for tablet formulation and further analyze its effect on the drug release of the SLS printed tablets under a specially designed magnetic field.
Methods: For the formulation of the model tablets, Carbonyl iron is an FDA-approved iron supplement (Code of Federal Regulations CFR Title 21); it was added to the formulation due to its oxidation resistance property, a good conductor of heat, and its magnetic property. For all the formulations, 200 g of a mixture of the model drug and excipients were blended using a mortar and pestle and transferred to the powder reservoir compartment (110x110x110 mm) of the SLS printer. The formulation for optimization and drug release analysis is referred to as F1. F1 is the standard formulation used for drug release studies. Compositions of carbonyl iron: API (isoniazid): Kollidon® VA64 (3:5:98) are hereafter referred to as F2. F3 refers to the formulation of powder blends with Candurin® : isoniazid: Kollidon® VA64 (3:5:98). F2 and F3 were used for comparing the quality of the sintering agents. During the printing process, the powder was spread like a layer of 0.15 mm by the roller and sintered by a 2.3 Watt blue diode laser (445 nm) at a selected scanning speed. The tablets were formed by sintering the powder layer-by-layer based on the designed STL file. After the printing process, the printer was cooled down. The tablets were collected from the powder bed after removing the loose powder.
Results: Sintering enhancers are important ingredients for the formulations of the SLS PTs since they determine the formation speed as a function of absorbed energy from the laser and the quality of pharmaceutical dosage forms. Candurin® is a potent colorant and hence until now, it has only been approved as an additive at a concentration of 1.25% in tablets and capsules. In this study, the results proved that without the help of the pigments type enhancer, 3 w/w% carbonyl iron shows its ability to improve the sintering quality of the PTs. The SLS PTs which contain 3 w/w% carbonyl iron, formed harder tablets than those containing 3 w/w of Candurin® Gold Sheen. Here, carbonyl irons were considered a new kind of sintering enhancer for SLS PTs. Also, compared with the pigments, using carbonyl iron as a sintering enhancer has another benefit which is it is a human iron supplement. For the SLS PT, the low scanning speed with tight hatching space results in thermal deformations. Furthermore, a large hatching space leads to incomplete sintering. Providing sufficient energy for the adequate bonding of the consecutive printing layers while maintaining the desired shape and dimensions of the printed tablets is essential. This study also fabricates a system that can control drug release by using the magnetic field. PLM images show that part of API dissolves into the VA64, but not like hot-melt extrusion printed tablets (strong mixing), API is rich on the surface of the SLS PTs. When the tablets came in contact with the dissolution media, regardless of the magnetic fields, the tablets disintegrated. Under the influence of a magnetic stimulus, the iron particles pass through the polymers and generate a microporous structure in PT. The dissolution study is aimed to prove that the carbonyl iron (magnetic iron particles) speeds up the drug release process. Also, under the magnetic field, printed tablets with carbonyl iron released 25% more drugs compared to those without. It can be claimed that magnetic nanoparticles appear as an alternative conductive material to facilitate the sintering process during SLS 3DP of dosage forms and open numerous opportunities for potential magnetically triggerable drug delivery systems.
Conclusion: An advanced drug formulation that contains carbonyl iron for making pharmaceutical tablets utilizing the SLS 3DP method was successfully manufactured. Carbonyl iron not only absorbed the laser energy efficiently, leading to successful sintering of the tablets but also helped to improve the release of the drug under the magnetic field by harnessing its magnetism. Furthermore, carbonyl iron is an FDA-approved iron supplement. When patients take the tablets, it helps them to replenish the daily required iron as well. Simultaneously, SLS 3DP provides a novel way to prepare an oral pharmaceutical dosage form in a single step. This method also provides a facile approach to tailor the quality of tablets by adjusting the tablet formulations as well as the printing parameters. This is achieved by adjusting the laser scanning speed, the hatching spacing, and the internal temperature.
References: Thakkar, R.; Zhang, Y.; Zhang, J.; Maniruzzaman, M. Synergistic application of twin-screw granulation and selective laser sintering 3D printing for the development of pharmaceutical dosage forms with enhanced dissolution rates and physical properties. Eur. J. Pharm. Biopharm. 2021, 163, 141–156, doi:10.1016/j.ejpb.2021.03.016.
Zhang, J.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Microwave induced dielectric heating for the on-demand development of indomethacin amorphous solid dispersion tablets. J. Drug Deliv. Sci. Technol. 2020, 102109, doi:https://doi.org/10.1016/j.jddst.2020.102109.
Acknowledgements: The research work reported herein was supported by Maniruzaman’s start-up funds at The University of Texas at Austin and the Faculty Science and Technology Acquisition and Retention (STARs) Award.
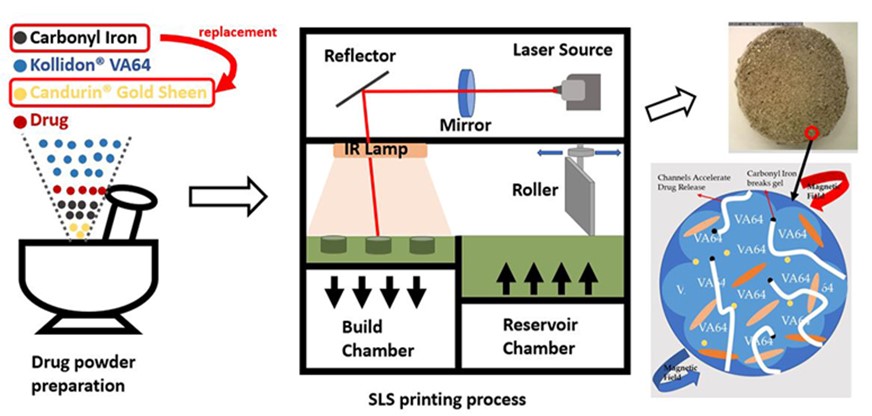
The schematic images of SLS sintering iron pharmaceutical dosages.

Digital microscope images of ideal PTs with different formulations (left) F1, (mid) F2, and (right) F3. The printing parameters are the same which are HS 13 µm, LSS 100 mm/s, and temperature 65 °C. Microscope images show its morphology. The diameter of the PTs were set up as 11.13 mm (Standard oral drug dosage form size).
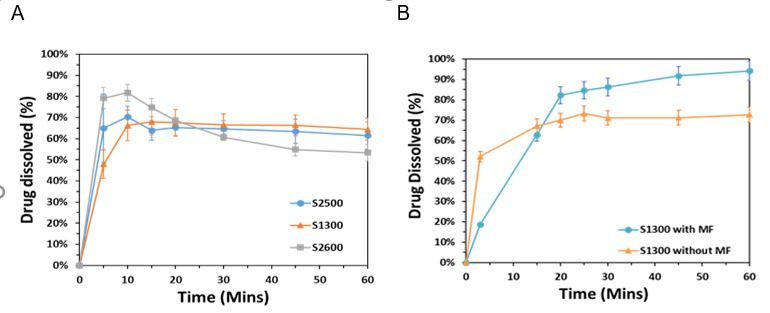
Dissolution profiles of Kollidon® VA 64 based formulation, (a) S1300, S2500, S2600, (b) S1300 samples with and without magnetic stimuli.
Discovery and Basic Research - Pharmaceutics
Category: Poster Abstract
(M1330-05-28) How a Sintering Enhancer Works as Multifunctional Ingredients for Selective Laser Sintering (SLS) 3D Printing of Pharmaceutical Dosage Forms
Monday, October 17, 2022
1:30 PM – 2:30 PM ET
- YZ
Yu Zhang, Ph.D.
University of Texas at Austin
Austin, Texas, United States - YZ
Yu Zhang, Ph.D.
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: Selective laser sintering (SLS) is a single-step three-dimensional printing (3DP) process that is gaining momentum in pharmaceutical dosage form manufacturing. This novel 3DP process offers opportunities for manufacturing various pharmaceutical dosage forms with a wide array of drug delivery systems. The purpose of this research was to introduce carbonyl iron as a multi-functional magnetic and heat conductive ingredient for tablet formulation and further analyze its effect on the drug release of the SLS printed tablets under a specially designed magnetic field.
Methods: For the formulation of the model tablets, Carbonyl iron is an FDA-approved iron supplement (Code of Federal Regulations CFR Title 21); it was added to the formulation due to its oxidation resistance property, a good conductor of heat, and its magnetic property. For all the formulations, 200 g of a mixture of the model drug and excipients were blended using a mortar and pestle and transferred to the powder reservoir compartment (110x110x110 mm) of the SLS printer. The formulation for optimization and drug release analysis is referred to as F1. F1 is the standard formulation used for drug release studies. Compositions of carbonyl iron: API (isoniazid): Kollidon® VA64 (3:5:98) are hereafter referred to as F2. F3 refers to the formulation of powder blends with Candurin® : isoniazid: Kollidon® VA64 (3:5:98). F2 and F3 were used for comparing the quality of the sintering agents. During the printing process, the powder was spread like a layer of 0.15 mm by the roller and sintered by a 2.3 Watt blue diode laser (445 nm) at a selected scanning speed. The tablets were formed by sintering the powder layer-by-layer based on the designed STL file. After the printing process, the printer was cooled down. The tablets were collected from the powder bed after removing the loose powder.
Results: Sintering enhancers are important ingredients for the formulations of the SLS PTs since they determine the formation speed as a function of absorbed energy from the laser and the quality of pharmaceutical dosage forms. Candurin® is a potent colorant and hence until now, it has only been approved as an additive at a concentration of 1.25% in tablets and capsules. In this study, the results proved that without the help of the pigments type enhancer, 3 w/w% carbonyl iron shows its ability to improve the sintering quality of the PTs. The SLS PTs which contain 3 w/w% carbonyl iron, formed harder tablets than those containing 3 w/w of Candurin® Gold Sheen. Here, carbonyl irons were considered a new kind of sintering enhancer for SLS PTs. Also, compared with the pigments, using carbonyl iron as a sintering enhancer has another benefit which is it is a human iron supplement. For the SLS PT, the low scanning speed with tight hatching space results in thermal deformations. Furthermore, a large hatching space leads to incomplete sintering. Providing sufficient energy for the adequate bonding of the consecutive printing layers while maintaining the desired shape and dimensions of the printed tablets is essential. This study also fabricates a system that can control drug release by using the magnetic field. PLM images show that part of API dissolves into the VA64, but not like hot-melt extrusion printed tablets (strong mixing), API is rich on the surface of the SLS PTs. When the tablets came in contact with the dissolution media, regardless of the magnetic fields, the tablets disintegrated. Under the influence of a magnetic stimulus, the iron particles pass through the polymers and generate a microporous structure in PT. The dissolution study is aimed to prove that the carbonyl iron (magnetic iron particles) speeds up the drug release process. Also, under the magnetic field, printed tablets with carbonyl iron released 25% more drugs compared to those without. It can be claimed that magnetic nanoparticles appear as an alternative conductive material to facilitate the sintering process during SLS 3DP of dosage forms and open numerous opportunities for potential magnetically triggerable drug delivery systems.
Conclusion: An advanced drug formulation that contains carbonyl iron for making pharmaceutical tablets utilizing the SLS 3DP method was successfully manufactured. Carbonyl iron not only absorbed the laser energy efficiently, leading to successful sintering of the tablets but also helped to improve the release of the drug under the magnetic field by harnessing its magnetism. Furthermore, carbonyl iron is an FDA-approved iron supplement. When patients take the tablets, it helps them to replenish the daily required iron as well. Simultaneously, SLS 3DP provides a novel way to prepare an oral pharmaceutical dosage form in a single step. This method also provides a facile approach to tailor the quality of tablets by adjusting the tablet formulations as well as the printing parameters. This is achieved by adjusting the laser scanning speed, the hatching spacing, and the internal temperature.
References: Thakkar, R.; Zhang, Y.; Zhang, J.; Maniruzzaman, M. Synergistic application of twin-screw granulation and selective laser sintering 3D printing for the development of pharmaceutical dosage forms with enhanced dissolution rates and physical properties. Eur. J. Pharm. Biopharm. 2021, 163, 141–156, doi:10.1016/j.ejpb.2021.03.016.
Zhang, J.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Microwave induced dielectric heating for the on-demand development of indomethacin amorphous solid dispersion tablets. J. Drug Deliv. Sci. Technol. 2020, 102109, doi:https://doi.org/10.1016/j.jddst.2020.102109.
Acknowledgements: The research work reported herein was supported by Maniruzaman’s start-up funds at The University of Texas at Austin and the Faculty Science and Technology Acquisition and Retention (STARs) Award.

The schematic images of SLS sintering iron pharmaceutical dosages.

Digital microscope images of ideal PTs with different formulations (left) F1, (mid) F2, and (right) F3. The printing parameters are the same which are HS 13 µm, LSS 100 mm/s, and temperature 65 °C. Microscope images show its morphology. The diameter of the PTs were set up as 11.13 mm (Standard oral drug dosage form size).

Dissolution profiles of Kollidon® VA 64 based formulation, (a) S1300, S2500, S2600, (b) S1300 samples with and without magnetic stimuli.
