Back
Purpose: The aim of the present study is to investigate about the LER effect observed in biological sample solution and the corrective actions applied to troubleshoot the phenomenon. The term “Low Endotoxin Recovery” (LER) describes the failure to detect spiked endotoxin in some sterile biological drug products when tested using the internationally harmonized compendial Limulus amebocyte lysate (LAL) assay. The inability to recover spiked endotoxins from finished drug products suggested that endotoxin contamination from the manufacturing processes may not be detected at release, thus, pyrogenic products could be distributed for commercial use in patients (4).
Methods: Turbidimetric Kinetic Method (1;2;3)
Results: A Drug Product (DP) composed of 2.0 mg/mL monoclonal antibody solubilized in buffer sodium phosphate dibasic 11.05 mg/mL and citric acid anhydrous 5.65 mg/mL was manufactured in a GMP area (Sterile suite) and analyzed for Bacteria Endotoxins using turbidimetric kinetic assay. 1:600 is the Dilution Required to Overcome (DROI) the Interfering factors according to the method suitability performed for the DP formulation challenged in the present study. The product was tested to determine the maximum sample storage time (Holding Time Study) prior to Bacterial Endotoxin Test (BET) take into consideration two different sample containers: depyrogenated flask to collect In-Process Control (IPC) sample and vial for the final DP. 2 EU/mL of Reference Standard Endotoxin (RSE) was spiked directly into the undiluted sample solutions (IPC sample and final DP) and tested at different checkpoints at dilution 1:600. During the study, all the samples were stored at Room Temperature (20-25 °C). A LER effect (spike recovery < 50%) was observed at time zero for IPC sample and finished DP diluted in LAL Reagent Water (Table 1 – Assay occasion 1). Different experimental sessions (Table 2 – Assay occasion 2) were carried out in order to overcome the LER effect using Endosafe Cation Buffer Solution (0.5 M of MgSO4 in 1M Tris) at different concentrations: 12.5mM of MgSO4, 15mM of MgSO4, 20mM of MgSO4 and 25mM of MgSO4. In addition, the sample mixing time was reduced from 30 seconds to 10 seconds increasing the theoretical RSE spike up to 8 EU/mL.
Conclusion: Endotoxins are complex lipopolysaccharides (LPS) which form an inherent fraction of the outer cell wall of all Gram negative bacteria and are responsible for the organization and stability of the cell wall. The presence of chelator agents (such as citric acid) into the sample solution, may destabilized the salt bridges between LPS and divalent cations (e.g., Mg2+, Ca2+), leading to reduced rigidity of the LPS aggregate causing LER effect. In LPS stabilized by salt bridges, LER may thus be induced by the interplay of a chelator (e.g., citrate) and a surfactant (e.g., polysorbate), but is not limited to these substances. Other molecules with the ability to interfere with the salt bridge between LPS molecules and alter the aggregation state can cause masking as well, for example, the protein itself (e.g., monoclonal antibody). As the interaction between the endotoxin and the protein can either be hydrophobic or ionic, the protein on its own can also be responsible for alteration of aggregation state of LPS. In such a case, a complex-forming buffer or surfactant would not be required to induce LER. Further side-by-side comparison studies have indicated that the LER effect is substantially enhanced by excessive vortex mixing (4). The increase of the RSE spike was adopted in order to obtain an endotoxin amount near to the middle point of the standard curve when the sample solution tested was diluted to 1:600. The results showed in table 2 (assay occasion 2) reveal compliant endotoxin recovery (i.e., > 50%) up to 15 days for the finished DP and up to 72 hours for the IPC sample despite what observed in assay occasion 1 (ref. Image 1).
References: 1. United State Pharmacopoeia USP - Bacterial Endotoxin Test
2. United State Pharmacopoeia USP - Guidelines on Endotoxins Test
3. European Pharmacopoeia EP 2.6.14 – Bacterial Endotoxins
4. PDA Technical Report No. 82 – Low Endotoxin Recovery
Acknowledgements: Thanks to all the personnel employed in QC Microbiological Laboratory of Patheon, part of Thermo Fisher Scientific, site located in via Morolense n. 5 Ferentino (FR) Italy
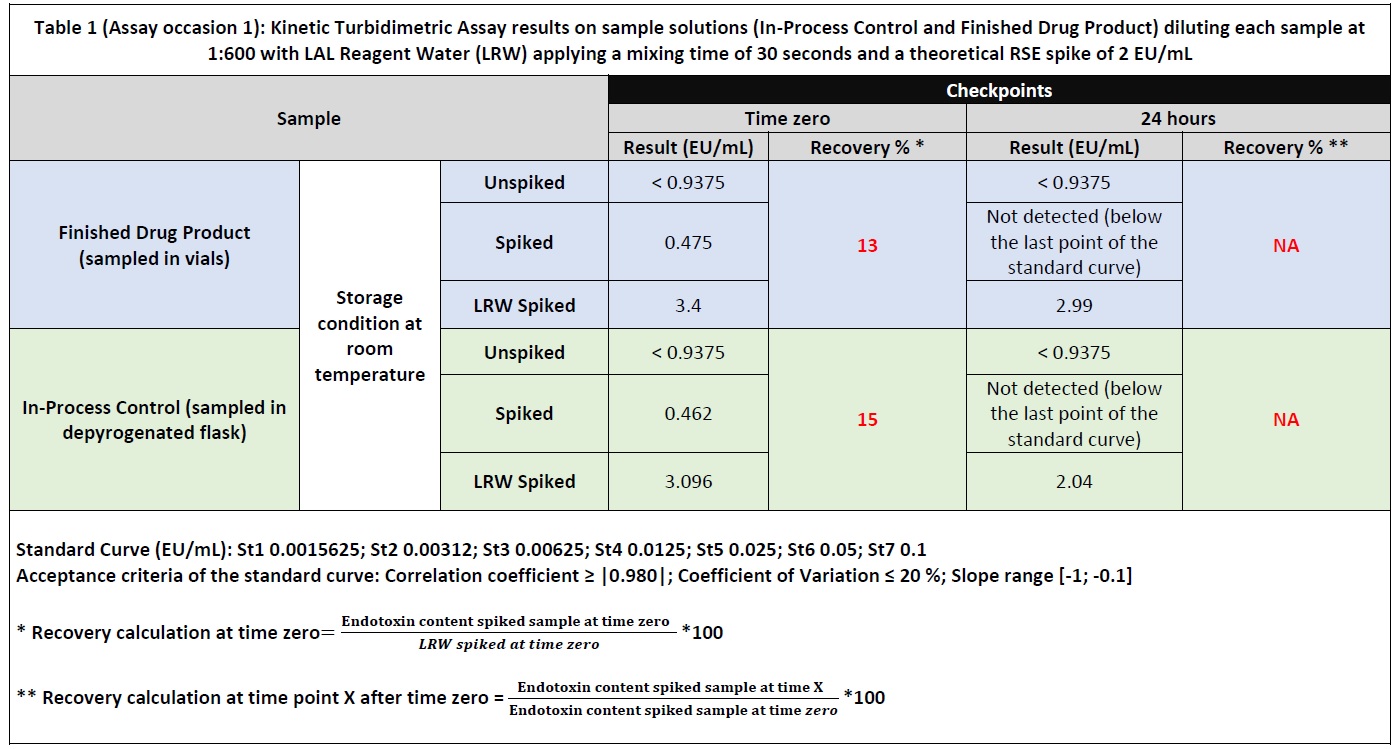
Table 1 (Assay occasion 1): Kinetic Turbidimetric Assay results on sample solutions (In-Process Control and Finished Drug Product) diluting each sample at 1:600 with LAL Reagent Water (LRW) applyng a mixing time of 30 seconds and a theoretical RSE spike of 2 EU/mL
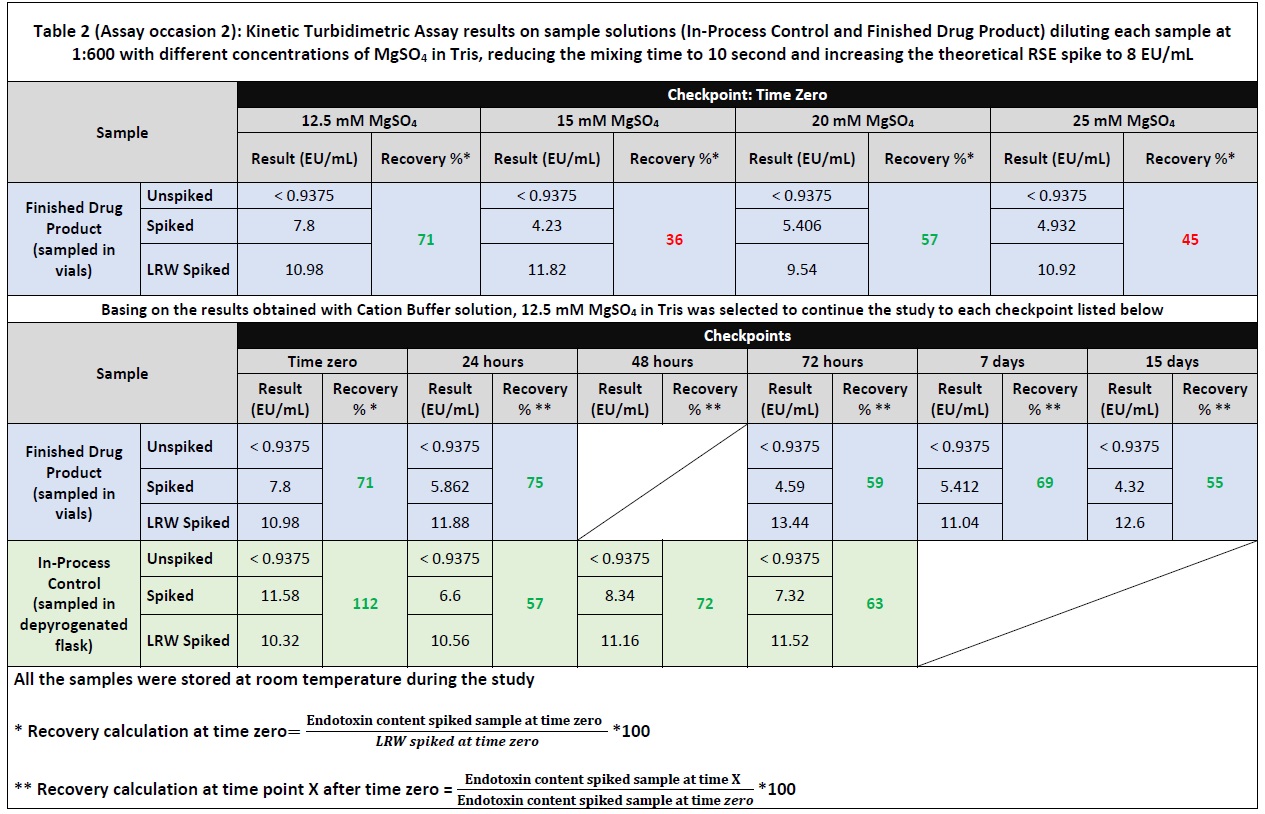
Assay occasion 2: Kinetic Turbidimetric Assay results on sample solutions (In-Process Control and Finished Drug Product) diluting each sample at 1:600 with LRW and different concentration of MgSO4 in Tris, reducing the mixing time to 10 seconds and increasing the theoretical RSE spike to 8 EU/mL
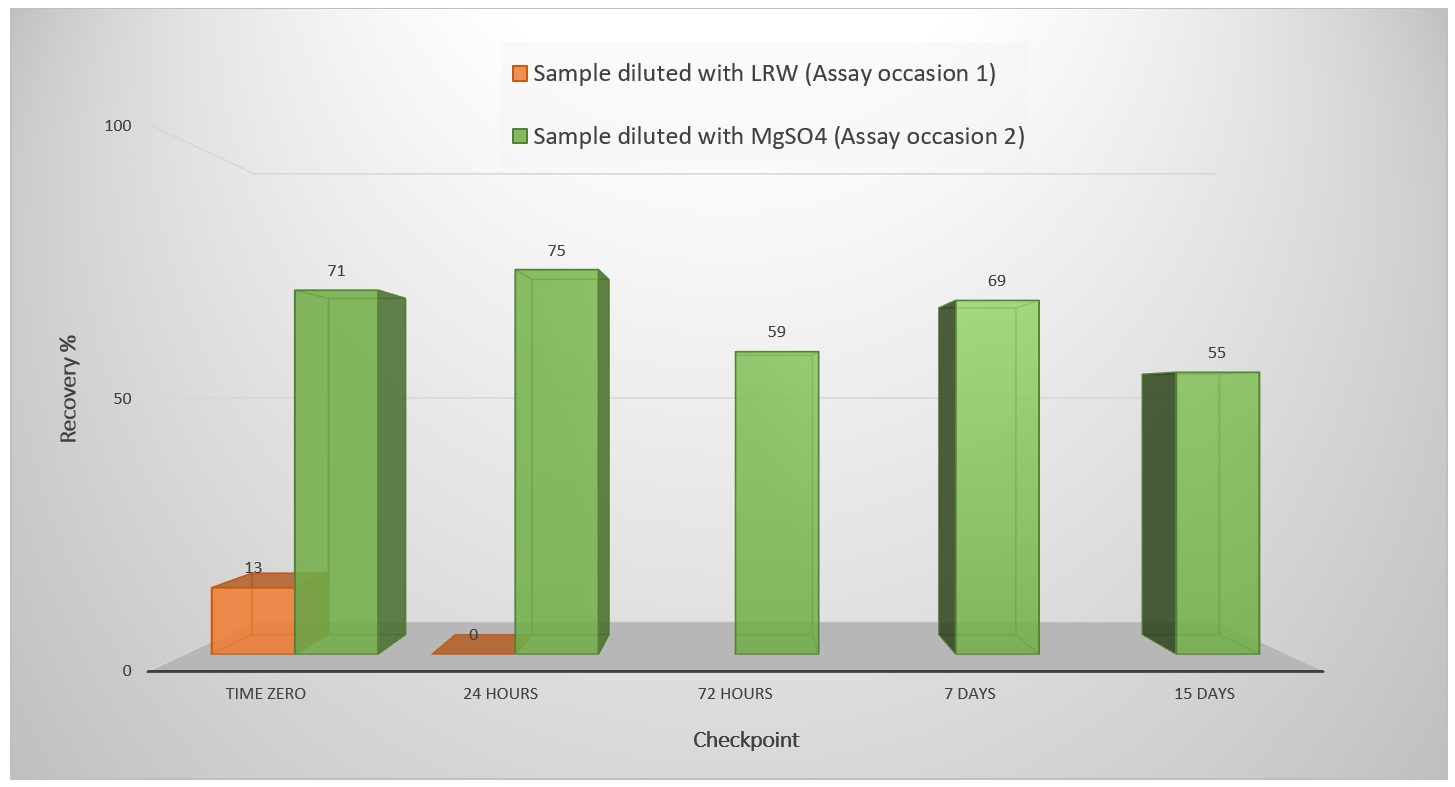
Image 1: RSE Spike recovery for finished DP for each checkpoint during assay occasion 1 and 2
Formulation and Delivery - Biomolecular - Administration
Category: Poster Abstract
(M0930-04-22) Low Endotoxin Recovery (LER) Effect in Sample Solution of Monoclonal Antibody in Sodium Phosphate Dibasic and Citric Acid Anhydrous Constitution Buffer Analyzed with LAL Turbidimetric Kinetic Assay
Monday, October 17, 2022
9:30 AM – 10:30 AM ET
- GP
Giulia Paterno, MS
Thermo Fisher Scientific
Ferentino, Lazio, Italy - CB
Carlo Bottoni, Ph.D.
Thermo Fisher Scientific
Ferentino, Lazio, Italy
Presenting Author(s)
Main Author(s)
Purpose: The aim of the present study is to investigate about the LER effect observed in biological sample solution and the corrective actions applied to troubleshoot the phenomenon. The term “Low Endotoxin Recovery” (LER) describes the failure to detect spiked endotoxin in some sterile biological drug products when tested using the internationally harmonized compendial Limulus amebocyte lysate (LAL) assay. The inability to recover spiked endotoxins from finished drug products suggested that endotoxin contamination from the manufacturing processes may not be detected at release, thus, pyrogenic products could be distributed for commercial use in patients (4).
Methods: Turbidimetric Kinetic Method (1;2;3)
Results: A Drug Product (DP) composed of 2.0 mg/mL monoclonal antibody solubilized in buffer sodium phosphate dibasic 11.05 mg/mL and citric acid anhydrous 5.65 mg/mL was manufactured in a GMP area (Sterile suite) and analyzed for Bacteria Endotoxins using turbidimetric kinetic assay. 1:600 is the Dilution Required to Overcome (DROI) the Interfering factors according to the method suitability performed for the DP formulation challenged in the present study. The product was tested to determine the maximum sample storage time (Holding Time Study) prior to Bacterial Endotoxin Test (BET) take into consideration two different sample containers: depyrogenated flask to collect In-Process Control (IPC) sample and vial for the final DP. 2 EU/mL of Reference Standard Endotoxin (RSE) was spiked directly into the undiluted sample solutions (IPC sample and final DP) and tested at different checkpoints at dilution 1:600. During the study, all the samples were stored at Room Temperature (20-25 °C). A LER effect (spike recovery < 50%) was observed at time zero for IPC sample and finished DP diluted in LAL Reagent Water (Table 1 – Assay occasion 1). Different experimental sessions (Table 2 – Assay occasion 2) were carried out in order to overcome the LER effect using Endosafe Cation Buffer Solution (0.5 M of MgSO4 in 1M Tris) at different concentrations: 12.5mM of MgSO4, 15mM of MgSO4, 20mM of MgSO4 and 25mM of MgSO4. In addition, the sample mixing time was reduced from 30 seconds to 10 seconds increasing the theoretical RSE spike up to 8 EU/mL.
Conclusion: Endotoxins are complex lipopolysaccharides (LPS) which form an inherent fraction of the outer cell wall of all Gram negative bacteria and are responsible for the organization and stability of the cell wall. The presence of chelator agents (such as citric acid) into the sample solution, may destabilized the salt bridges between LPS and divalent cations (e.g., Mg2+, Ca2+), leading to reduced rigidity of the LPS aggregate causing LER effect. In LPS stabilized by salt bridges, LER may thus be induced by the interplay of a chelator (e.g., citrate) and a surfactant (e.g., polysorbate), but is not limited to these substances. Other molecules with the ability to interfere with the salt bridge between LPS molecules and alter the aggregation state can cause masking as well, for example, the protein itself (e.g., monoclonal antibody). As the interaction between the endotoxin and the protein can either be hydrophobic or ionic, the protein on its own can also be responsible for alteration of aggregation state of LPS. In such a case, a complex-forming buffer or surfactant would not be required to induce LER. Further side-by-side comparison studies have indicated that the LER effect is substantially enhanced by excessive vortex mixing (4). The increase of the RSE spike was adopted in order to obtain an endotoxin amount near to the middle point of the standard curve when the sample solution tested was diluted to 1:600. The results showed in table 2 (assay occasion 2) reveal compliant endotoxin recovery (i.e., > 50%) up to 15 days for the finished DP and up to 72 hours for the IPC sample despite what observed in assay occasion 1 (ref. Image 1).
References: 1. United State Pharmacopoeia USP - Bacterial Endotoxin Test
2. United State Pharmacopoeia USP - Guidelines on Endotoxins Test
3. European Pharmacopoeia EP 2.6.14 – Bacterial Endotoxins
4. PDA Technical Report No. 82 – Low Endotoxin Recovery
Acknowledgements: Thanks to all the personnel employed in QC Microbiological Laboratory of Patheon, part of Thermo Fisher Scientific, site located in via Morolense n. 5 Ferentino (FR) Italy

Table 1 (Assay occasion 1): Kinetic Turbidimetric Assay results on sample solutions (In-Process Control and Finished Drug Product) diluting each sample at 1:600 with LAL Reagent Water (LRW) applyng a mixing time of 30 seconds and a theoretical RSE spike of 2 EU/mL

Assay occasion 2: Kinetic Turbidimetric Assay results on sample solutions (In-Process Control and Finished Drug Product) diluting each sample at 1:600 with LRW and different concentration of MgSO4 in Tris, reducing the mixing time to 10 seconds and increasing the theoretical RSE spike to 8 EU/mL

Image 1: RSE Spike recovery for finished DP for each checkpoint during assay occasion 1 and 2
