Back
Purpose: Malignant mesothelioma (MM) is a rare type of cancer that accounts for ~30,000 new cases worldwide annually (1). MM is characterized by tumors found at the lining of major organs such as lungs, heart, and abdomen. Malignant pleural mesothelioma (MPM), or cancer primarily affecting mesothelial cells lining the lungs accounts for 80-90% of all MM cases. Occupational exposure of asbestos fibers has been identified as the major cause of MPM that requires 10-50 years for the symptoms to develop (2). From a therapeutic perspective, conventional chemotherapy, surgical procedures, radiation, as well as immunotherapy has not been very successful, and the patients experience a plethora of adverse side effects with < 10% 5-year survival rate (3). As a result, there is an increased urgency for scientists to develop more efficacious treatment options while minimizing the possible side effects. In our project, we selected celastrol (Cela), an active phytochemical constituent studied extensively in variety of diseases including applications as an antioxidant and an anti-tumor agent (4). However, translation of Cela to clinical applications remains challenging due to limitations in low aqueous solubility, poor permeability, undesirable biodistribution, and pharmacokinetic profile. To address these limitations, we propose pulmonary delivery via inhalation of celastrol-loaded PLGA microparticles (Cela MPs) to promote localized deposition in the lungs and enhanced therapeutic activity for the treatment of MPM.
Methods: Cela MPs were prepared using standard double emulsion solvent evaporation method, and were evaluated for particle size, PDI, zeta potential, and drug entrapment. Pore-forming agents or porogens such as NaCl and NaHCO₃ were incorporated into the formulations to introduce a wrinkled or porous particle morphology. Physical characterization studies including SEM, DSC, XRD, release were performed. In-vitro aerosolization behavior of the microparticles was characterized using Next Generation Impactor (NGI). Cytotoxic potential was quantified in 4 mesothelioma cell lines following 48-h drug incubation using MTT. A chronic 15-day 3D spheroid study was implemented to mimic in-vivo conditions, to simulate preclinical efficacy. MSTO-211H cells were seeded onto spheroid plates for 3 days. Thereafter, spheroids were treated with varying concentrations of Cela and Cela MPs, with media as control. Imaging of spheroids was captured on day 0,1, 3, 6, 9, 12, and 15. For single dose study, half the media was replaced with fresh media on each imaging day, while half the media was replaced with corresponding concentrations of treatments for multiple dose study. DPPH and caspase-3 study were done to test antioxidant and apoptotic activity.
Results: Table 1 summarizes the various formulations developed for this project. F6 was ultimately selected as the optimized formulation with highest % entrapment efficiency of 72.8±6.1% (or 2.4±0.1% drug loading), ideal particle size (PS) of 2,076±390 nm, PDI of 0.3±0.2, and a stable zeta potential of -36.6±3.8mV. DSC and XRD studies indicated successful encapsulation of Cela into the microparticles. SEM images taken confirmed the microparticle PS ~2µm with a wrinkled surface morphology (Fig. 1A). The particle deposition (%) of MP on each stage of the NGI is plotted in Fig. 1B, along with the cumulative deposition (%) as a function of effective cut-off diameter is represented in Fig. 1C. A large collection of particles belonging in stage 3 and below which represent bronchoalveolar deposition. Calculated mass median aerodynamic diameter (MMAD) from NGI study were 4.5±0.1 µm, with GSD of 2.1±0.1µm and % FPF of 85.3±2.5% (Fig. 1D), suggesting excellent aerosolization properties of Cela MPs. Cytotoxicity studies showed Cela MPs significantly reduced IC50 values compared to plain Cela on all four mesothelioma cell lines tested. 3D spheroid volume analysis showed significant antitumor efficacy of Cela MPs compared to control and plain Cela (Fig. 2). Specifically, a single dose of Cela MPs at 1.0 µM was sufficient to considerably inhibit spheroid growth throughout the 15-day study. DPPH antioxidant assay indicated MPs retained antioxidant activity of drug, and caspase-3 study showed MPs at 1.0 µM induced higher apoptosis compared to other treatments.
Conclusion: In conclusion, these studies highlight the anti-mesothelioma efficacy of Cela MPs at low doses with excellent aerosolization properties for pulmonary delivery. While preliminary results are promising, further preclinical studies will be conducted to perceive the full potential of this approach.
References: 1. Zhai Z, Ruan J, Zheng Y, Xiang D, Li N, Hu J, et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw Open. 2021 Aug 11;4(8):e2120360.
2. Malignant Mesothelioma Cancer | Stages, Prognosis, Treatment [Internet]. Mesothelioma.com. [cited 2022 May 2]. Available from: https://www.mesothelioma.com/mesothelioma/
3. Survival Rates for Mesothelioma [Internet]. [cited 2022 May 2]. Available from: https://www.cancer.org/cancer/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html
4. Shukla SK, Chan A, Parvathaneni V, Kanabar DD, Patel K, Ayehunie S, et al. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J Mol Liq. 2020 Nov;318:113936.
Acknowledgements: The authors would like to acknowledge the Imaging Facility of CUNY Advanced Science Research Center for instrument use, scientific and technical assistance.
Funding: This project was funded with the start-up funds to Vivek Gupta by College of Pharmacy and Health Sciences, St. John’s University, Queens, NY. Xuechun Wang and Gautam Chauhan were supported with the teaching assistantships by St. John’s University.
Conflict of Interest: All authors declare no conflict of interest.
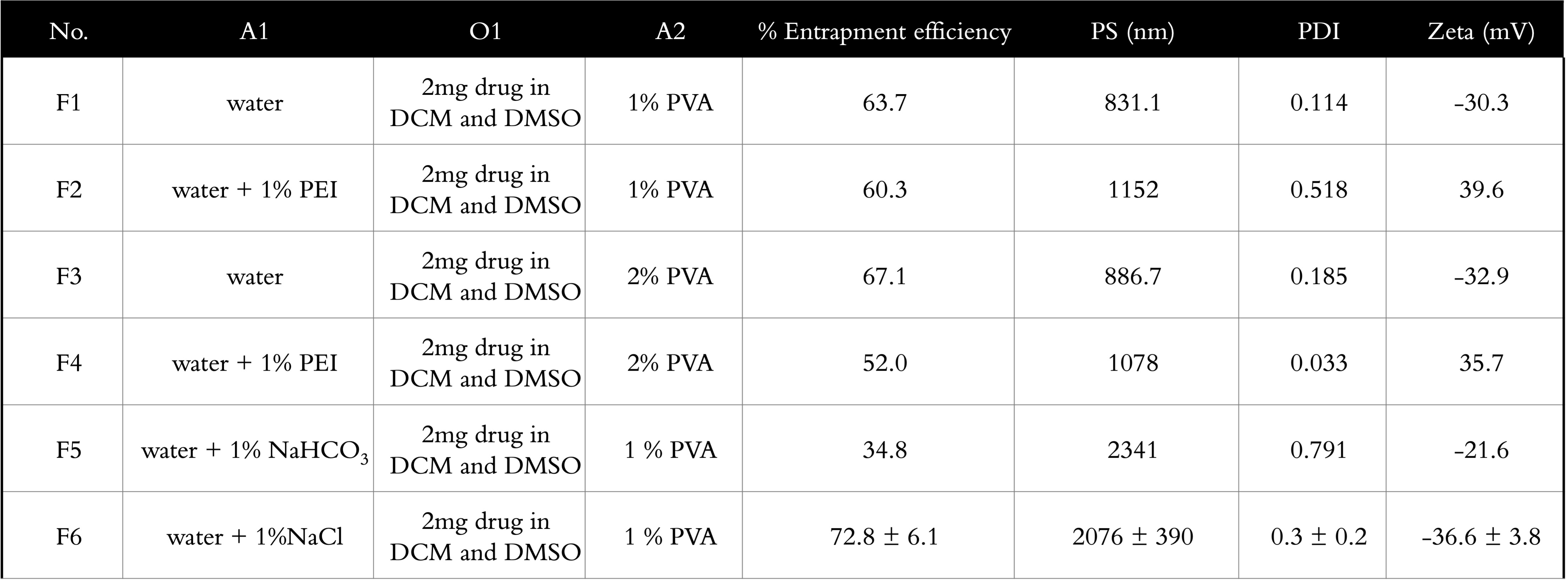
Table 1: Summary of formulation optimization for Cela MPs. F6 was chosen as the optimized formulation for further studies. A1: inner aqueous phase. O1: organic phase. A2: external aqueous phase.
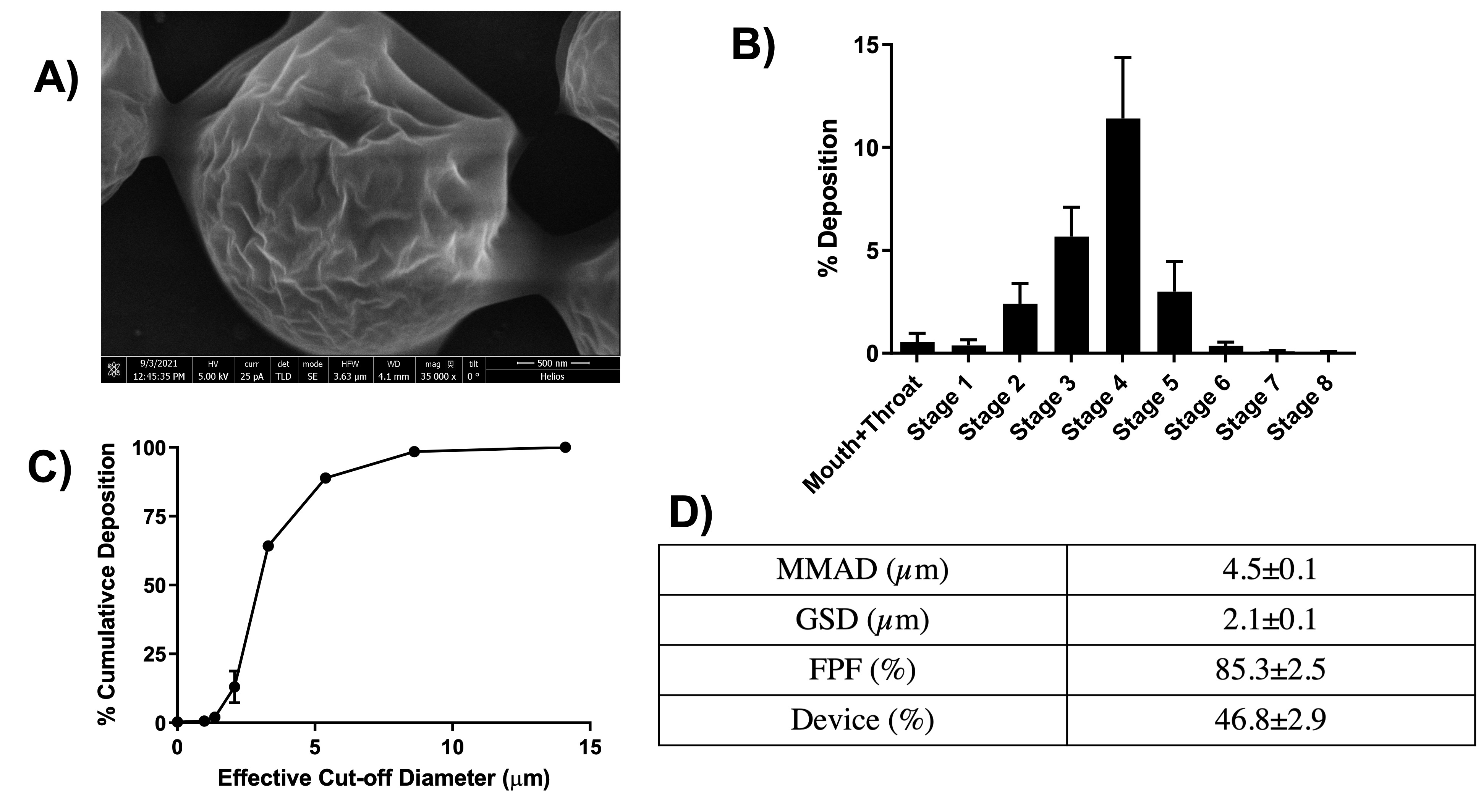
Figure 1: A. SEM image of Cela MP and aerodynamic performance of Cela MPs. B. % deposition of Cela MPs in various stages of Next-Gen ImpactorTM (NGI), C. % cumulative deposition as a function of effective cut-off diameter of Cela MPs. D. Summary of aerosolization parameters obtained and calculated from using NGI. Data represent mean±SD (n=3).
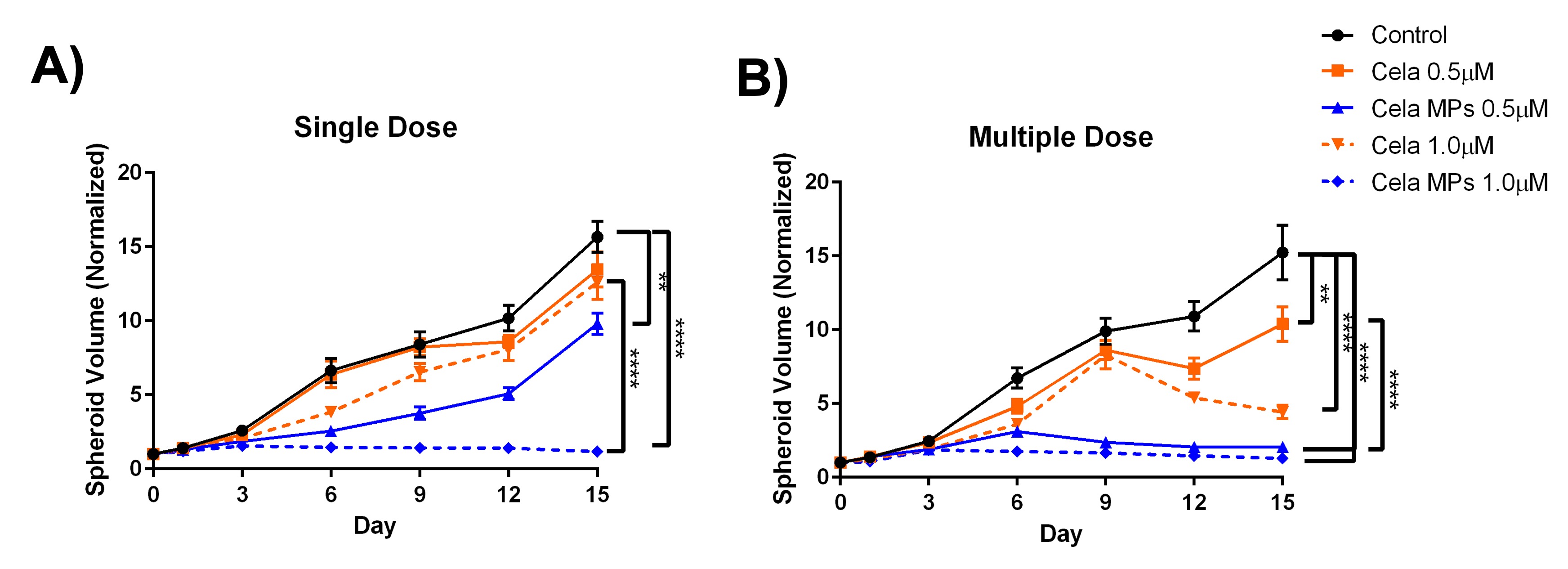
Figure 2: 3D spheroid study to determine effect of treatments on MSTO-211H tumor spheroids growth. Quantification of normalized spheroid volume over 15 days was compared between treatments after either single dose (A) or multiple dose (B). Data represent mean ± SD (n=6). Significance between the groups was analyzed by one-way ANOVA and Tukey’s multiple comparisons test. **p < 0.01 and ****p < 0.0001.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M0930-07-41) Surface-Modified Inhalable Microparticle-Encapsulated Triterpenoid for Enhanced Efficacy in Malignant Mesothelioma
Monday, October 17, 2022
9:30 AM – 10:30 AM ET
- XW
Xuechun Wang, BS
St. John's University
Jamaica, New York, United States - XW
Xuechun Wang, BS
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Malignant mesothelioma (MM) is a rare type of cancer that accounts for ~30,000 new cases worldwide annually (1). MM is characterized by tumors found at the lining of major organs such as lungs, heart, and abdomen. Malignant pleural mesothelioma (MPM), or cancer primarily affecting mesothelial cells lining the lungs accounts for 80-90% of all MM cases. Occupational exposure of asbestos fibers has been identified as the major cause of MPM that requires 10-50 years for the symptoms to develop (2). From a therapeutic perspective, conventional chemotherapy, surgical procedures, radiation, as well as immunotherapy has not been very successful, and the patients experience a plethora of adverse side effects with < 10% 5-year survival rate (3). As a result, there is an increased urgency for scientists to develop more efficacious treatment options while minimizing the possible side effects. In our project, we selected celastrol (Cela), an active phytochemical constituent studied extensively in variety of diseases including applications as an antioxidant and an anti-tumor agent (4). However, translation of Cela to clinical applications remains challenging due to limitations in low aqueous solubility, poor permeability, undesirable biodistribution, and pharmacokinetic profile. To address these limitations, we propose pulmonary delivery via inhalation of celastrol-loaded PLGA microparticles (Cela MPs) to promote localized deposition in the lungs and enhanced therapeutic activity for the treatment of MPM.
Methods: Cela MPs were prepared using standard double emulsion solvent evaporation method, and were evaluated for particle size, PDI, zeta potential, and drug entrapment. Pore-forming agents or porogens such as NaCl and NaHCO₃ were incorporated into the formulations to introduce a wrinkled or porous particle morphology. Physical characterization studies including SEM, DSC, XRD, release were performed. In-vitro aerosolization behavior of the microparticles was characterized using Next Generation Impactor (NGI). Cytotoxic potential was quantified in 4 mesothelioma cell lines following 48-h drug incubation using MTT. A chronic 15-day 3D spheroid study was implemented to mimic in-vivo conditions, to simulate preclinical efficacy. MSTO-211H cells were seeded onto spheroid plates for 3 days. Thereafter, spheroids were treated with varying concentrations of Cela and Cela MPs, with media as control. Imaging of spheroids was captured on day 0,1, 3, 6, 9, 12, and 15. For single dose study, half the media was replaced with fresh media on each imaging day, while half the media was replaced with corresponding concentrations of treatments for multiple dose study. DPPH and caspase-3 study were done to test antioxidant and apoptotic activity.
Results: Table 1 summarizes the various formulations developed for this project. F6 was ultimately selected as the optimized formulation with highest % entrapment efficiency of 72.8±6.1% (or 2.4±0.1% drug loading), ideal particle size (PS) of 2,076±390 nm, PDI of 0.3±0.2, and a stable zeta potential of -36.6±3.8mV. DSC and XRD studies indicated successful encapsulation of Cela into the microparticles. SEM images taken confirmed the microparticle PS ~2µm with a wrinkled surface morphology (Fig. 1A). The particle deposition (%) of MP on each stage of the NGI is plotted in Fig. 1B, along with the cumulative deposition (%) as a function of effective cut-off diameter is represented in Fig. 1C. A large collection of particles belonging in stage 3 and below which represent bronchoalveolar deposition. Calculated mass median aerodynamic diameter (MMAD) from NGI study were 4.5±0.1 µm, with GSD of 2.1±0.1µm and % FPF of 85.3±2.5% (Fig. 1D), suggesting excellent aerosolization properties of Cela MPs. Cytotoxicity studies showed Cela MPs significantly reduced IC50 values compared to plain Cela on all four mesothelioma cell lines tested. 3D spheroid volume analysis showed significant antitumor efficacy of Cela MPs compared to control and plain Cela (Fig. 2). Specifically, a single dose of Cela MPs at 1.0 µM was sufficient to considerably inhibit spheroid growth throughout the 15-day study. DPPH antioxidant assay indicated MPs retained antioxidant activity of drug, and caspase-3 study showed MPs at 1.0 µM induced higher apoptosis compared to other treatments.
Conclusion: In conclusion, these studies highlight the anti-mesothelioma efficacy of Cela MPs at low doses with excellent aerosolization properties for pulmonary delivery. While preliminary results are promising, further preclinical studies will be conducted to perceive the full potential of this approach.
References: 1. Zhai Z, Ruan J, Zheng Y, Xiang D, Li N, Hu J, et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw Open. 2021 Aug 11;4(8):e2120360.
2. Malignant Mesothelioma Cancer | Stages, Prognosis, Treatment [Internet]. Mesothelioma.com. [cited 2022 May 2]. Available from: https://www.mesothelioma.com/mesothelioma/
3. Survival Rates for Mesothelioma [Internet]. [cited 2022 May 2]. Available from: https://www.cancer.org/cancer/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html
4. Shukla SK, Chan A, Parvathaneni V, Kanabar DD, Patel K, Ayehunie S, et al. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J Mol Liq. 2020 Nov;318:113936.
Acknowledgements: The authors would like to acknowledge the Imaging Facility of CUNY Advanced Science Research Center for instrument use, scientific and technical assistance.
Funding: This project was funded with the start-up funds to Vivek Gupta by College of Pharmacy and Health Sciences, St. John’s University, Queens, NY. Xuechun Wang and Gautam Chauhan were supported with the teaching assistantships by St. John’s University.
Conflict of Interest: All authors declare no conflict of interest.

Table 1: Summary of formulation optimization for Cela MPs. F6 was chosen as the optimized formulation for further studies. A1: inner aqueous phase. O1: organic phase. A2: external aqueous phase.

Figure 1: A. SEM image of Cela MP and aerodynamic performance of Cela MPs. B. % deposition of Cela MPs in various stages of Next-Gen ImpactorTM (NGI), C. % cumulative deposition as a function of effective cut-off diameter of Cela MPs. D. Summary of aerosolization parameters obtained and calculated from using NGI. Data represent mean±SD (n=3).

Figure 2: 3D spheroid study to determine effect of treatments on MSTO-211H tumor spheroids growth. Quantification of normalized spheroid volume over 15 days was compared between treatments after either single dose (A) or multiple dose (B). Data represent mean ± SD (n=6). Significance between the groups was analyzed by one-way ANOVA and Tukey’s multiple comparisons test. **p < 0.01 and ****p < 0.0001.
