Back
Purpose: Transdermal administration of a non-steroidal anti-inflammatory drug (NSAID) is highly recommended over oral NSAID consumption for chronic conditions like arthritis pain[1]. Delivering the right amount of NSAID in a sustainable fashion for an extended time using a more patient-compliant dosage form is desired. This study was conducted to determine the suitability of silicone and acrylic pressure sensitive adhesive (PSA) blends to deliver a model drug, diclofenac base (DCF), sustainably at varying levels using matrix-type transdermal patch formulations. The benefit of utilizing PSA blends over individual PSAs was investigated for efficient DCF delivery and for patch adhesive characteristics.
Methods: Amine compatible silicone PSA, non-functional acrylic PSA, ethoxyethoxyethanol, butyl alcohol, and oleyl alcohol were used to make matrix type patch formulations with a varying ratio of silicone to acrylic PSA while keeping other excipients at the same level. The DCF was incorporated at 2% w/w in the final non-volatile formulations. The formulation, initially containing ethyl acetate (carrier solvent for both silicone and acrylic PSAs), was cast at 18 mil on a polyester film and devolatilized at room temperature for 30 min followed by 5 minutes at 70°C to remove the ethyl acetate. Upon cooling, the patch laminates were covered with a fluoropolymer release liner. The preparation provided a 6 mil thick patch containing 0.35 mg/cm2 DCF. The in-vitro permeability experiments were conducted for 48 hr at 32°C in Franz cell apparatus using heat separated human cadaver epidermis as a membrane and phosphate buffered saline (PBS) as the receptor fluid. The amount of drug that permeated through the skin was determined using ultra performance liquid chromatography (UPLC). A small amount of formulation still in solvent was applied on top of a microscope glass slide to form a thin layer, dried at room temperature and then at 70°C to remove ethyl acetate. The slide was observed under a microscope equipped with a polarizing filter to evaluate drug crystallization in the matrix. Tack measurements for the laminate strips were measured using texture analyzer in quadruplicate using a stainless-steel probe with a contact diameter of 7 mm and applying 100 g of load to evaluate adhesive properties.
Results: All formulations except the one prepared using 100% acrylic PSA were translucent viscous liquids. The ratio of the silicone to acrylic PSA in the formulations impacted the DCF delivery profile non-linearly. Patch formulations with silicone: acrylic ratios of 100:0, 95:5, 90:10, 85:15, 75:25, 50:50, 25:75 and 0:100 delivered cumulative DCF amount of 96.0±26, 88.5±33, 103.7±15, 122.1±16, 112.7±36, 65.2±32, 58.8±33 and 53.9±20 mg respectively in 48 hr. The formulation with silicone: acrylic ratios of 85:15 and 75:25 delivered about 4 mg/cm2/hr of DCF nearly sustainably starting from about 5 hr and then through the entire 48 hr study (Fig 1) which was higher than that by any other formulation. The formulation with 100% silicone delivered a high amount of DCF at the initial period, from 3-12 hr, however, this level of delivery was not sustained for the remainder of the time. The formulation with 100% acrylic PSA delivered the least amount of DCF among all the formulations investigated. Examination of formulations under the microscope disclosed the presence of some DCF crystals for the formulation made with 100% silicone PSA (Fig 2) but not for any other formulations. DCF might have been near its saturation which could have assisted high DCF delivery at the initial period, but recrystallization could have led to less DCF delivery during the later period. The presence of acrylic PSA might have increased DCF solubility and compatibility with other excipients. However, when evaluating the patch formulation made only with acrylic PSA, DCF delivery was significantly decreased; demonstrating the enhanced delivery benefits provided by the silicone PSA. Tack measurements showed a moderate increase in the peak force, however, significant increase in the work of adhesion representing increased softness of the patch formulation (Fig 3) when acrylic PSA was incorporated.
Conclusion: The matrix transdermal patch formulations containing DCF were prepared and investigated for efficient DCF delivery and adhesive tack performance. The patch formulation containing a silicone:acrylic PSA blend ratio of 85:15 was found as performing best in terms of drug delivery and adhesion compared to other formulations.
References: [1]. F.B. Revel, M. Fayet, M. Hagen, Topical diclofenac, an efficacious treatment for osteoarthritis: A narrative review, Rheumatol. Ther. 2020, 7, 217-236
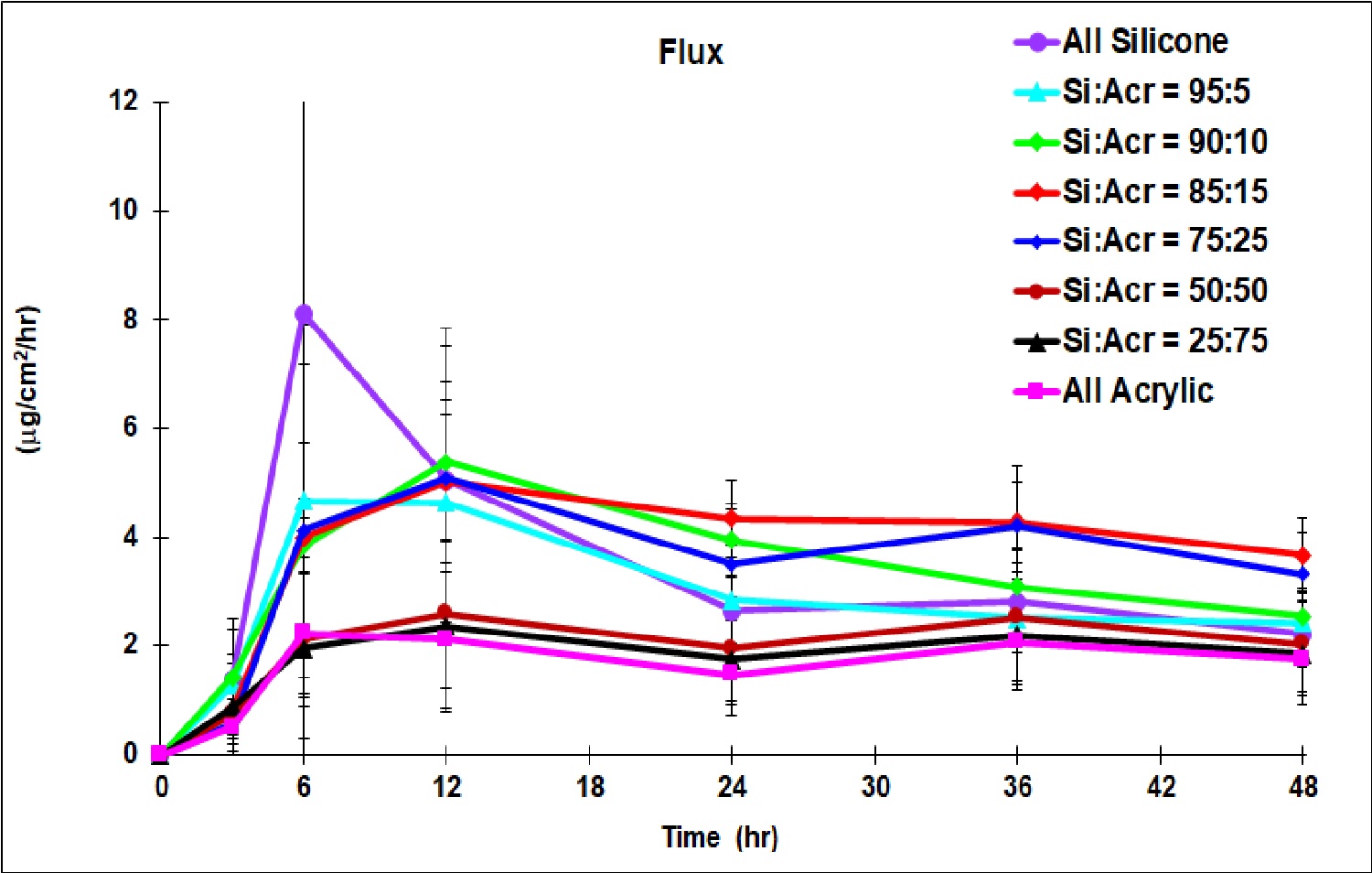
Fig. 1. DCF permeability profiles from silicone-acrylic patch formulations
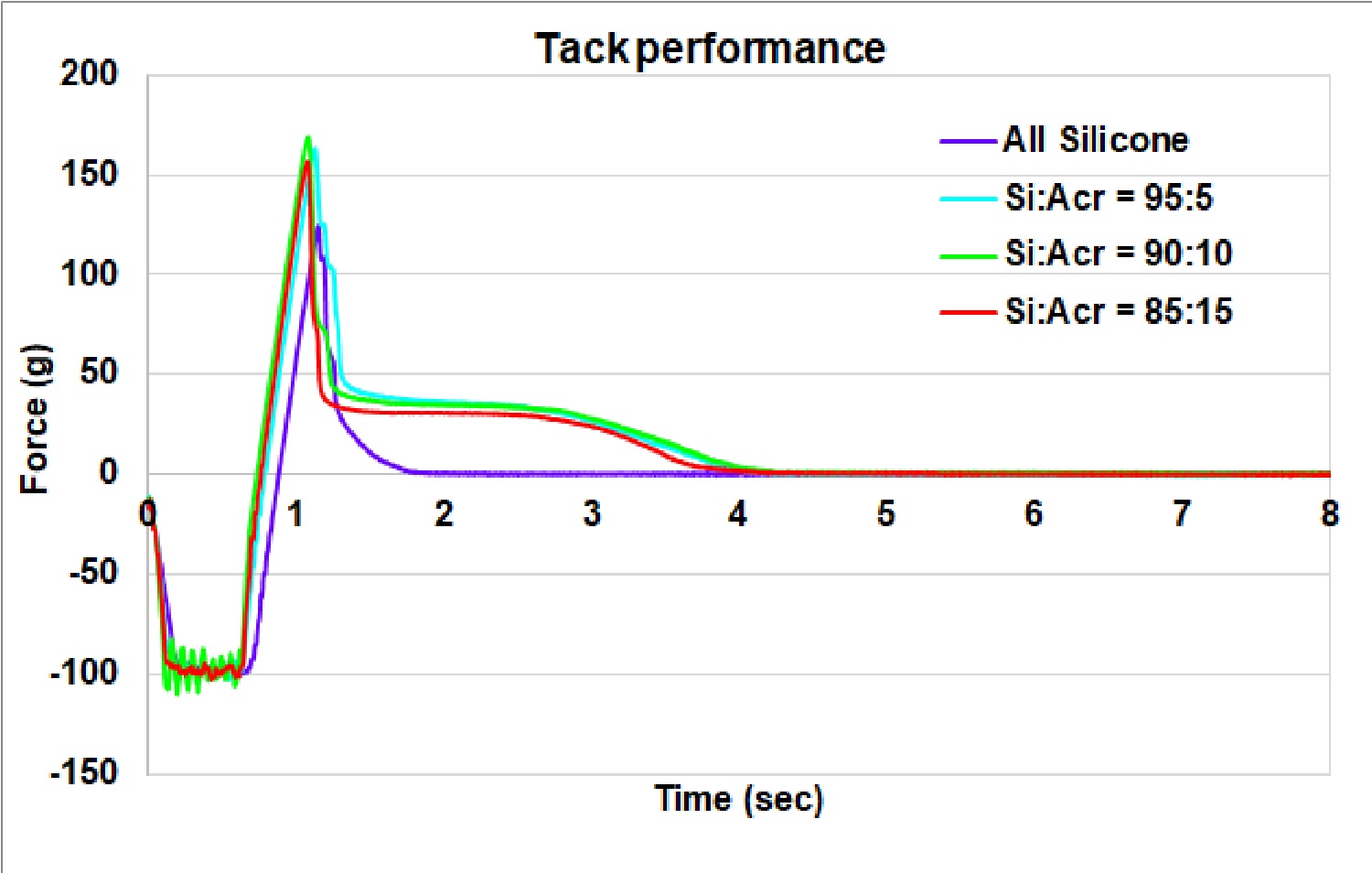
Fig. 2. Adhesion characteristics of patch formulations
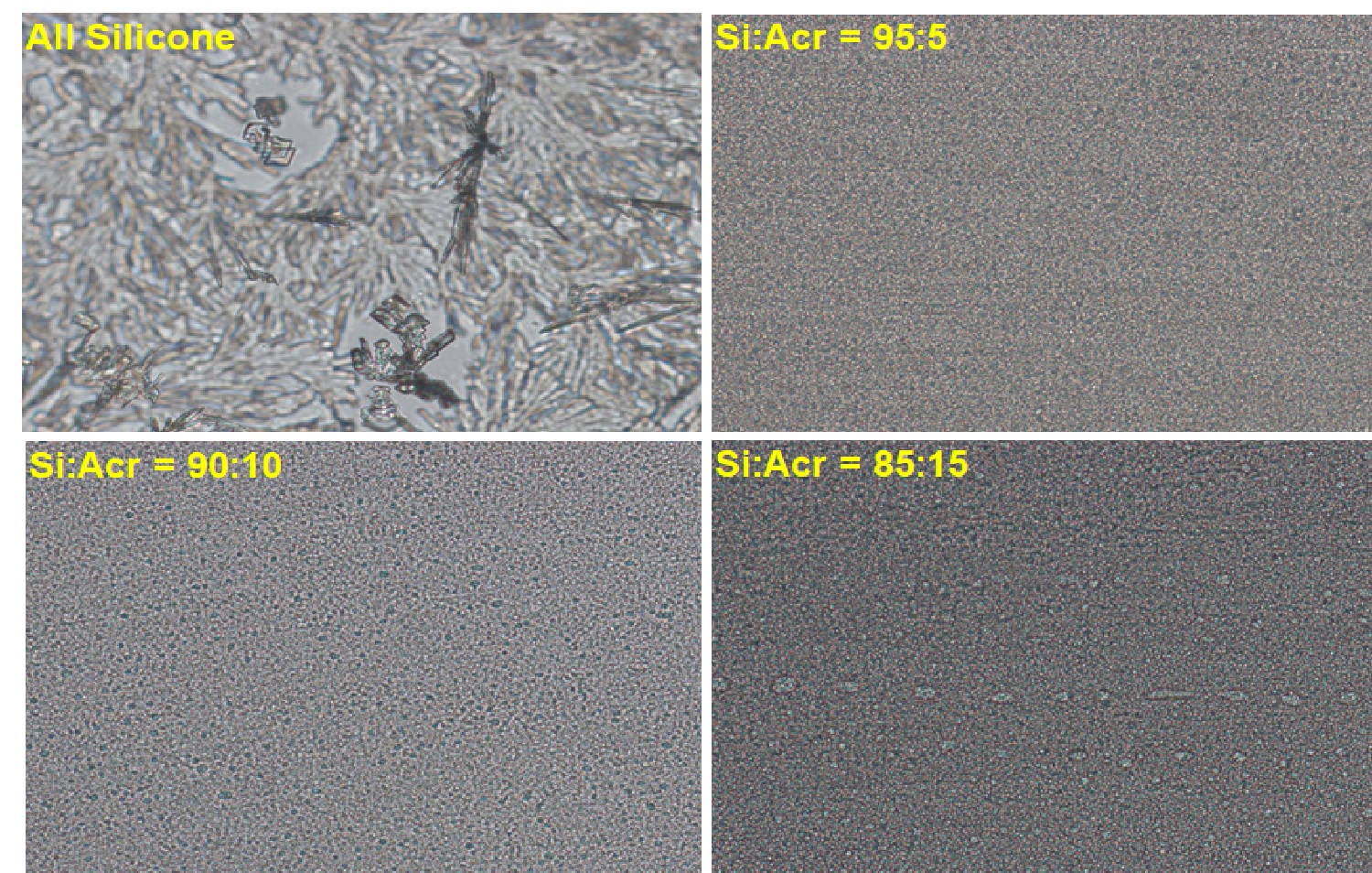
Fig. 3. Optical microscopic observation using plane polarized light with 200X magnification
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(W1230-07-38) Transdermal Delivery of Diclofenac Base: Effect of Silicone and Acrylic Pressure Sensitive Adhesive Blends on Drug Delivery and Adhesive Performance
Wednesday, October 19, 2022
12:30 PM – 1:30 PM ET

Hyder Aliyar, PhD
Lead Scientist
DuPont
Midland, Michigan, United States
Hyder Aliyar, PhD
Lead Scientist
DuPont
Midland, Michigan, United States
Presenting Author(s)
Main Author(s)
Purpose: Transdermal administration of a non-steroidal anti-inflammatory drug (NSAID) is highly recommended over oral NSAID consumption for chronic conditions like arthritis pain[1]. Delivering the right amount of NSAID in a sustainable fashion for an extended time using a more patient-compliant dosage form is desired. This study was conducted to determine the suitability of silicone and acrylic pressure sensitive adhesive (PSA) blends to deliver a model drug, diclofenac base (DCF), sustainably at varying levels using matrix-type transdermal patch formulations. The benefit of utilizing PSA blends over individual PSAs was investigated for efficient DCF delivery and for patch adhesive characteristics.
Methods: Amine compatible silicone PSA, non-functional acrylic PSA, ethoxyethoxyethanol, butyl alcohol, and oleyl alcohol were used to make matrix type patch formulations with a varying ratio of silicone to acrylic PSA while keeping other excipients at the same level. The DCF was incorporated at 2% w/w in the final non-volatile formulations. The formulation, initially containing ethyl acetate (carrier solvent for both silicone and acrylic PSAs), was cast at 18 mil on a polyester film and devolatilized at room temperature for 30 min followed by 5 minutes at 70°C to remove the ethyl acetate. Upon cooling, the patch laminates were covered with a fluoropolymer release liner. The preparation provided a 6 mil thick patch containing 0.35 mg/cm2 DCF. The in-vitro permeability experiments were conducted for 48 hr at 32°C in Franz cell apparatus using heat separated human cadaver epidermis as a membrane and phosphate buffered saline (PBS) as the receptor fluid. The amount of drug that permeated through the skin was determined using ultra performance liquid chromatography (UPLC). A small amount of formulation still in solvent was applied on top of a microscope glass slide to form a thin layer, dried at room temperature and then at 70°C to remove ethyl acetate. The slide was observed under a microscope equipped with a polarizing filter to evaluate drug crystallization in the matrix. Tack measurements for the laminate strips were measured using texture analyzer in quadruplicate using a stainless-steel probe with a contact diameter of 7 mm and applying 100 g of load to evaluate adhesive properties.
Results: All formulations except the one prepared using 100% acrylic PSA were translucent viscous liquids. The ratio of the silicone to acrylic PSA in the formulations impacted the DCF delivery profile non-linearly. Patch formulations with silicone: acrylic ratios of 100:0, 95:5, 90:10, 85:15, 75:25, 50:50, 25:75 and 0:100 delivered cumulative DCF amount of 96.0±26, 88.5±33, 103.7±15, 122.1±16, 112.7±36, 65.2±32, 58.8±33 and 53.9±20 mg respectively in 48 hr. The formulation with silicone: acrylic ratios of 85:15 and 75:25 delivered about 4 mg/cm2/hr of DCF nearly sustainably starting from about 5 hr and then through the entire 48 hr study (Fig 1) which was higher than that by any other formulation. The formulation with 100% silicone delivered a high amount of DCF at the initial period, from 3-12 hr, however, this level of delivery was not sustained for the remainder of the time. The formulation with 100% acrylic PSA delivered the least amount of DCF among all the formulations investigated. Examination of formulations under the microscope disclosed the presence of some DCF crystals for the formulation made with 100% silicone PSA (Fig 2) but not for any other formulations. DCF might have been near its saturation which could have assisted high DCF delivery at the initial period, but recrystallization could have led to less DCF delivery during the later period. The presence of acrylic PSA might have increased DCF solubility and compatibility with other excipients. However, when evaluating the patch formulation made only with acrylic PSA, DCF delivery was significantly decreased; demonstrating the enhanced delivery benefits provided by the silicone PSA. Tack measurements showed a moderate increase in the peak force, however, significant increase in the work of adhesion representing increased softness of the patch formulation (Fig 3) when acrylic PSA was incorporated.
Conclusion: The matrix transdermal patch formulations containing DCF were prepared and investigated for efficient DCF delivery and adhesive tack performance. The patch formulation containing a silicone:acrylic PSA blend ratio of 85:15 was found as performing best in terms of drug delivery and adhesion compared to other formulations.
References: [1]. F.B. Revel, M. Fayet, M. Hagen, Topical diclofenac, an efficacious treatment for osteoarthritis: A narrative review, Rheumatol. Ther. 2020, 7, 217-236

Fig. 1. DCF permeability profiles from silicone-acrylic patch formulations

Fig. 2. Adhesion characteristics of patch formulations

Fig. 3. Optical microscopic observation using plane polarized light with 200X magnification
