Back
Purpose: This study was conducted to determine the shelf-life of a tablet formulation containing amorphous spray-dried intermediate (SDI) consisting of an active pharmaceutical ingredient (API) and hypromellose acetate succinate polymer (HPMCAS-H) in a 30:70 ratio. The degradation kinetics for the main degradant peaks were also evaluated and the rate-limiting step was observed to be diffusion. The model is based on an isoconversion approach which is focused on the initial degradation rates up to the specification limit. Isoconversion is defined as the point of failure for the product. Two different specification limits were assessed in this study, 0.1% and 0.2%, and the effect of this change is reflected in the shelf-life predictions, particularly in the open storage conditions. To assess the effect of temperature and water on degradation, tablet samples were placed on stability, and the concentration of each degradant in the stressed samples were modeled using ASAPprime® software (version 6.0.1), and the moisture-modified Arrhenius equation, lnk=lnA-(Ea/RT)+B(RH). The following terms were calculated for six degradant peaks observed in the stressed tablet samples:
Methods: During the stability assessment, it is important to not cross any phase boundaries (e.g, glass transition temperature, Tg) as this significantly affects the linearity of degradation rates. As a result, the length of the study is primarily determined by the Tg and degree of water uptake by the SDI. A representative ASAP study design is shown in Table 1. The condition and timepoints are listed along with the appropriate saturated salt solution to maintain that humidity at the specified temperature. Samples were analyzed for impurities via HPLC, and these concentrations were input into the ASAPprime software and the resulting parameters, ln A, Ea and B terms, were compared.
Results: The calculated model parameters for the tablets in this study are shown in Table 2, and the shelf-life predictions are shown in Table 3. The activation energy term, Ea, reflects the temperature sensitivity for the isoconversion, and the B-term, reflects the moisture sensitivity for the isoconversion. For 3 out of the 4 degradant species modeled, the model parameters generated using 0.1% and 0.2% as the specification limit were within 10%. An exception was observed for the degradant species at RRT 1.054. For this species, the difference in the model parameters generated was 15%. This species is also identified as the shelf-life limiting species for this product as it had the lowest B term and the addition of desiccant did not have a significant effect on lengthening the shelf-life.
Conclusion: Stability studies using the moisture-modified Arrhenius model are being increasingly applied across the pharmaceutical industry to predict shelf-life and recommend packaging configurations. The recommended packaging configuration for the tablets is 30 units inside 30 cc HDPE/HIS bottles with 1g desiccant. This study also demonstrates the significance of selecting the specification limit to be used in the modeling, as the model is built on the isoconversion approach.
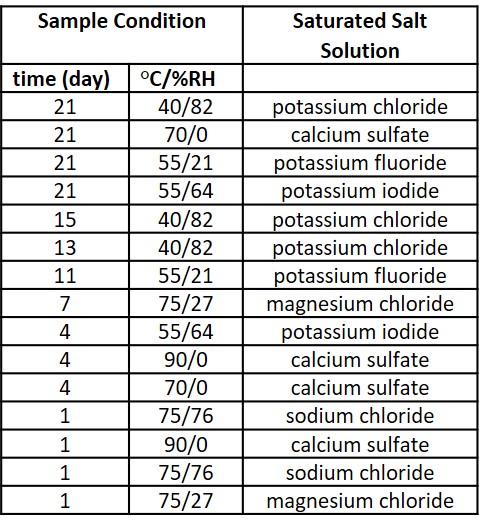
Table 1. Representative stability condition table showing timepoint, condition and saturated salt solution to maintain the condition.
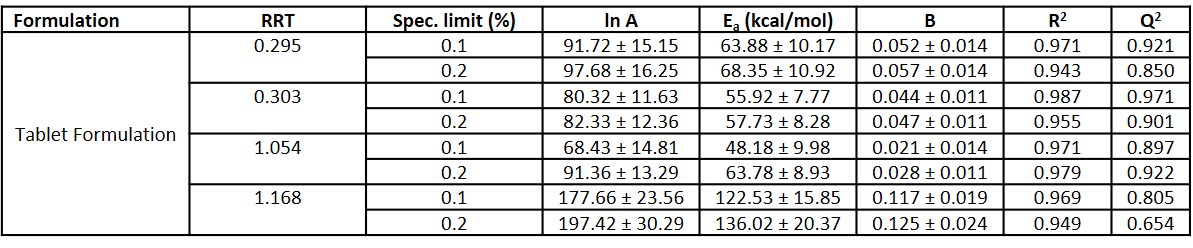
Table 2. Moisture-modified Arrhenius model parameters for Tablet Formulation
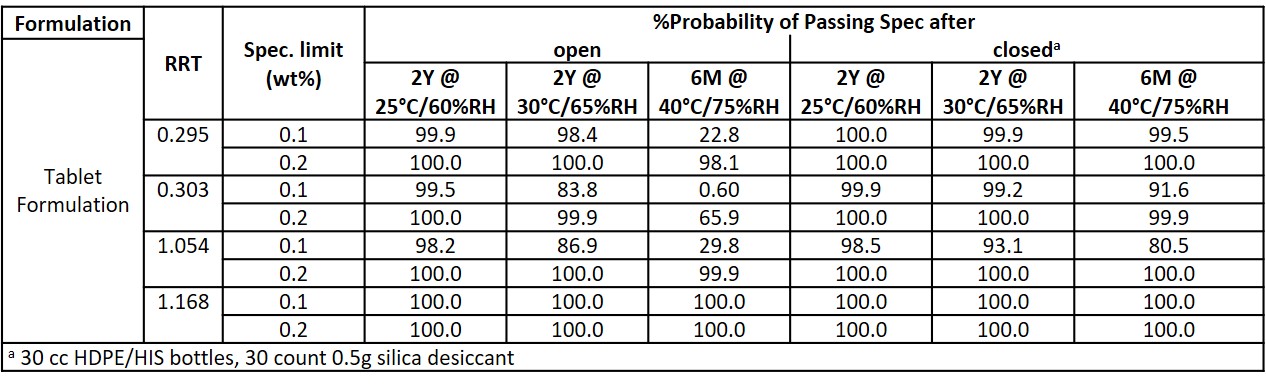
Table 3. Shelf-life Predictions for Tablet Formulation in open and closed packaging configurations.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(W1130-01-06) Amorphous Chemical Stability of an Oral Tablet Using Moisture-Modified Arrhenius Modeling
Wednesday, October 19, 2022
11:30 AM – 12:30 PM ET
- SB
Stephanie Buchanan, MS
Thermo Fisher Scientific
Bend, Oregon, United States - SB
Stephanie Buchanan, MS
Thermo Fisher Scientific
Bend, Oregon, United States
Presenting Author(s)
Main Author(s)
Purpose: This study was conducted to determine the shelf-life of a tablet formulation containing amorphous spray-dried intermediate (SDI) consisting of an active pharmaceutical ingredient (API) and hypromellose acetate succinate polymer (HPMCAS-H) in a 30:70 ratio. The degradation kinetics for the main degradant peaks were also evaluated and the rate-limiting step was observed to be diffusion. The model is based on an isoconversion approach which is focused on the initial degradation rates up to the specification limit. Isoconversion is defined as the point of failure for the product. Two different specification limits were assessed in this study, 0.1% and 0.2%, and the effect of this change is reflected in the shelf-life predictions, particularly in the open storage conditions. To assess the effect of temperature and water on degradation, tablet samples were placed on stability, and the concentration of each degradant in the stressed samples were modeled using ASAPprime® software (version 6.0.1), and the moisture-modified Arrhenius equation, lnk=lnA-(Ea/RT)+B(RH). The following terms were calculated for six degradant peaks observed in the stressed tablet samples:
- Ea (activation energy): reflects sensitivity to temperature,
- B: the humidity sensitivity factor, and
- ln A: the collision frequency.
Methods: During the stability assessment, it is important to not cross any phase boundaries (e.g, glass transition temperature, Tg) as this significantly affects the linearity of degradation rates. As a result, the length of the study is primarily determined by the Tg and degree of water uptake by the SDI. A representative ASAP study design is shown in Table 1. The condition and timepoints are listed along with the appropriate saturated salt solution to maintain that humidity at the specified temperature. Samples were analyzed for impurities via HPLC, and these concentrations were input into the ASAPprime software and the resulting parameters, ln A, Ea and B terms, were compared.
Results: The calculated model parameters for the tablets in this study are shown in Table 2, and the shelf-life predictions are shown in Table 3. The activation energy term, Ea, reflects the temperature sensitivity for the isoconversion, and the B-term, reflects the moisture sensitivity for the isoconversion. For 3 out of the 4 degradant species modeled, the model parameters generated using 0.1% and 0.2% as the specification limit were within 10%. An exception was observed for the degradant species at RRT 1.054. For this species, the difference in the model parameters generated was 15%. This species is also identified as the shelf-life limiting species for this product as it had the lowest B term and the addition of desiccant did not have a significant effect on lengthening the shelf-life.
Conclusion: Stability studies using the moisture-modified Arrhenius model are being increasingly applied across the pharmaceutical industry to predict shelf-life and recommend packaging configurations. The recommended packaging configuration for the tablets is 30 units inside 30 cc HDPE/HIS bottles with 1g desiccant. This study also demonstrates the significance of selecting the specification limit to be used in the modeling, as the model is built on the isoconversion approach.

Table 1. Representative stability condition table showing timepoint, condition and saturated salt solution to maintain the condition.

Table 2. Moisture-modified Arrhenius model parameters for Tablet Formulation

Table 3. Shelf-life Predictions for Tablet Formulation in open and closed packaging configurations.
