Back
Purpose: A spray-dried dispersion (SDD) is a well-established manufacturing technique used to prepare amorphous solid dispersions (ASDs), allowing for poorly soluble drugs to have improved bioavailability. However, the process of spray-drying with multiple factors and numerous variables can lead to a lengthy development timeline with intense resource requirements, which becomes the main obstacle limiting spray-drying development at the preclinical stage. The purpose of this work was to identify optimized preset parameters for spray-drying to support the early development of ASDs suitable for most circumstances rather than individual optimization. This work has since been published in AAPS PharmSciTech.
Methods: Spray-dried solid dispersions were generated with a BUCHI B-290 mini spray dryer. The sprays were conducted in a closed-loop configuration using the Inert Loop B-295 and secondary drying was applied to all batches using a vacuum oven. Inlet temperature was targeted as 100 to 105 °C while outlet temperature was targeted to be 60 to 65 °C. Thus, the delta temperature between the outlet and inlet was kept at 40 ± 5 °C. Spray solution feed rate was held constant (10 mL/min). Multiple solvent mixtures including Acetone:Methanol (90:10), Acetone:Water (90:10), and Dichloromethane:Methanol (50:50) were found to be compatible with these spray-drying conditions. First, a mini-DoE (Design of Experiment) study was designed to evaluate the critical interplay of two key variables for spray-drying: solid load and atomizing spray gas flow. The critical quality attributes (CQAs) of the ASDs, including yield, particle size, morphology, and in-vitro release profile, were taken into account to identify the impact of the key variables. Once these predefined values were determined they were further verified using different formulation compositions, including various polymers (Eudragit L100-55, HPMCAS-MF, PVAP, and PVP-VA64), multiple drugs, a range of drug loadings (10 to 40%), and various scales (200 mg to 200 g). Characterization techniques used for experimentation included pXRD, mDSC, PLM, PSD, and SEM. Particle dissolution and solubility were explored as well.
Results: As the most critical parameters atomization pressure and total solid content were first evaluated, while all other variables were held constant. The dispersion particles were then analyzed for size, morphology, and dissolution properties. It was determined that 5% solid load and 35 mm atomizing spray gas flow generated the optimal ASD with the highest recovery, adequate particle size, and satisfying in-vitro dissolution performance. With these critical parameters determined, different polymers (enteric and non-enteric) were evaluated using these spraying conditions. Yields and particle size values were found to be independent from the type of polymer used. When looking at varying the API, the set parameters also produced consistent particle sizes and yields. In addition, yield and particle size were found to be independent of drug load. Finally, when looking at varying the scale of the spray, particle size also remained consistent with similar D50 values.
Conclusion: From the mini-DoE study through the verification steps, this work demonstrated the capabilities of applying preset spray-drying parameters to formulation screening. In general, 5% solid load (formulation parameter) and 35 mm (0.3 bar) atomizing spray gas flow (process parameter) were applicable when using various polymers, drug loads, APIs, and batch sizes. All ASD formulations prepared using the preset parameters in this study demonstrated a consistent particle size distribution (D50 < 10 µm) and satisfying yields ( > 50%). It can be concluded that this approach can be beneficial in preclinical early-stage formulation development to reduce resource requirements and shorten the developmental timeline. This translatable and reproducible spray-drying platform can also help streamline ASD development, providing support for establishing a formulation for toxicology studies and being a valuable resource for developing a clinical formulation.
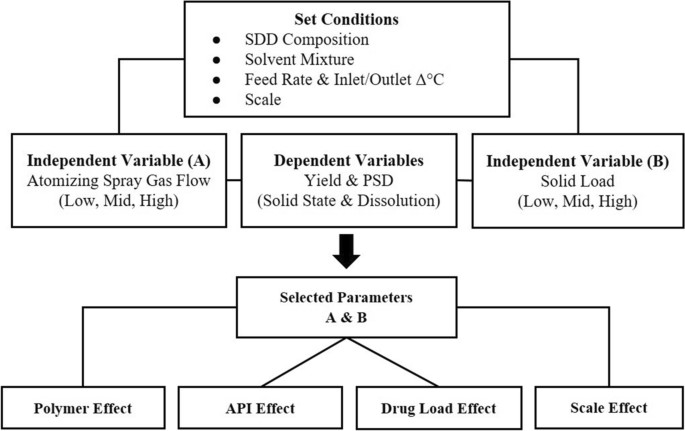
Figure 1: Workflow of ASD mini-DoE (top) and verification step (bottom)
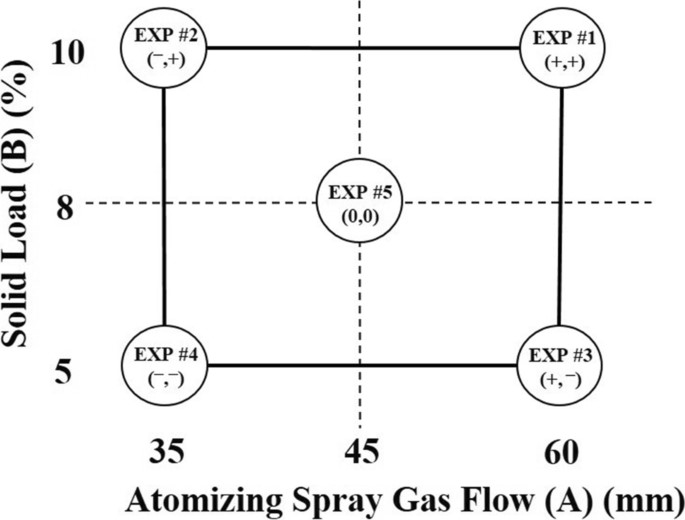
Figure 2: Mini-DoE of key independent variables
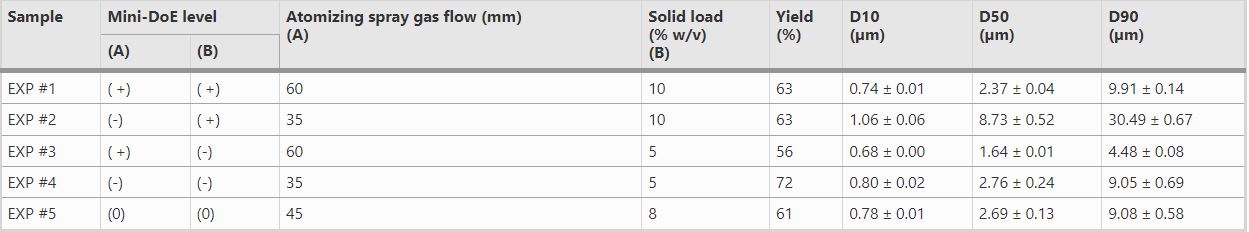
Table 1: Observed Effects of Atomizing Spray Gas Flow (A) and Solid Load (B)
Discovery and Basic Research - Pharmaceutics
Category: Late Breaking Poster Abstract
(W1030-05-27) Optimization of Spray-Drying Parameters for Formulation Development at Preclinical Scale
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- RK
Robert Kuhn, BS
PPD Laboratories
South San Francisco, California, United States - MN
Marika Nespi, MS
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Purpose: A spray-dried dispersion (SDD) is a well-established manufacturing technique used to prepare amorphous solid dispersions (ASDs), allowing for poorly soluble drugs to have improved bioavailability. However, the process of spray-drying with multiple factors and numerous variables can lead to a lengthy development timeline with intense resource requirements, which becomes the main obstacle limiting spray-drying development at the preclinical stage. The purpose of this work was to identify optimized preset parameters for spray-drying to support the early development of ASDs suitable for most circumstances rather than individual optimization. This work has since been published in AAPS PharmSciTech.
Methods: Spray-dried solid dispersions were generated with a BUCHI B-290 mini spray dryer. The sprays were conducted in a closed-loop configuration using the Inert Loop B-295 and secondary drying was applied to all batches using a vacuum oven. Inlet temperature was targeted as 100 to 105 °C while outlet temperature was targeted to be 60 to 65 °C. Thus, the delta temperature between the outlet and inlet was kept at 40 ± 5 °C. Spray solution feed rate was held constant (10 mL/min). Multiple solvent mixtures including Acetone:Methanol (90:10), Acetone:Water (90:10), and Dichloromethane:Methanol (50:50) were found to be compatible with these spray-drying conditions. First, a mini-DoE (Design of Experiment) study was designed to evaluate the critical interplay of two key variables for spray-drying: solid load and atomizing spray gas flow. The critical quality attributes (CQAs) of the ASDs, including yield, particle size, morphology, and in-vitro release profile, were taken into account to identify the impact of the key variables. Once these predefined values were determined they were further verified using different formulation compositions, including various polymers (Eudragit L100-55, HPMCAS-MF, PVAP, and PVP-VA64), multiple drugs, a range of drug loadings (10 to 40%), and various scales (200 mg to 200 g). Characterization techniques used for experimentation included pXRD, mDSC, PLM, PSD, and SEM. Particle dissolution and solubility were explored as well.
Results: As the most critical parameters atomization pressure and total solid content were first evaluated, while all other variables were held constant. The dispersion particles were then analyzed for size, morphology, and dissolution properties. It was determined that 5% solid load and 35 mm atomizing spray gas flow generated the optimal ASD with the highest recovery, adequate particle size, and satisfying in-vitro dissolution performance. With these critical parameters determined, different polymers (enteric and non-enteric) were evaluated using these spraying conditions. Yields and particle size values were found to be independent from the type of polymer used. When looking at varying the API, the set parameters also produced consistent particle sizes and yields. In addition, yield and particle size were found to be independent of drug load. Finally, when looking at varying the scale of the spray, particle size also remained consistent with similar D50 values.
Conclusion: From the mini-DoE study through the verification steps, this work demonstrated the capabilities of applying preset spray-drying parameters to formulation screening. In general, 5% solid load (formulation parameter) and 35 mm (0.3 bar) atomizing spray gas flow (process parameter) were applicable when using various polymers, drug loads, APIs, and batch sizes. All ASD formulations prepared using the preset parameters in this study demonstrated a consistent particle size distribution (D50 < 10 µm) and satisfying yields ( > 50%). It can be concluded that this approach can be beneficial in preclinical early-stage formulation development to reduce resource requirements and shorten the developmental timeline. This translatable and reproducible spray-drying platform can also help streamline ASD development, providing support for establishing a formulation for toxicology studies and being a valuable resource for developing a clinical formulation.

Figure 1: Workflow of ASD mini-DoE (top) and verification step (bottom)

Figure 2: Mini-DoE of key independent variables

Table 1: Observed Effects of Atomizing Spray Gas Flow (A) and Solid Load (B)
