Back
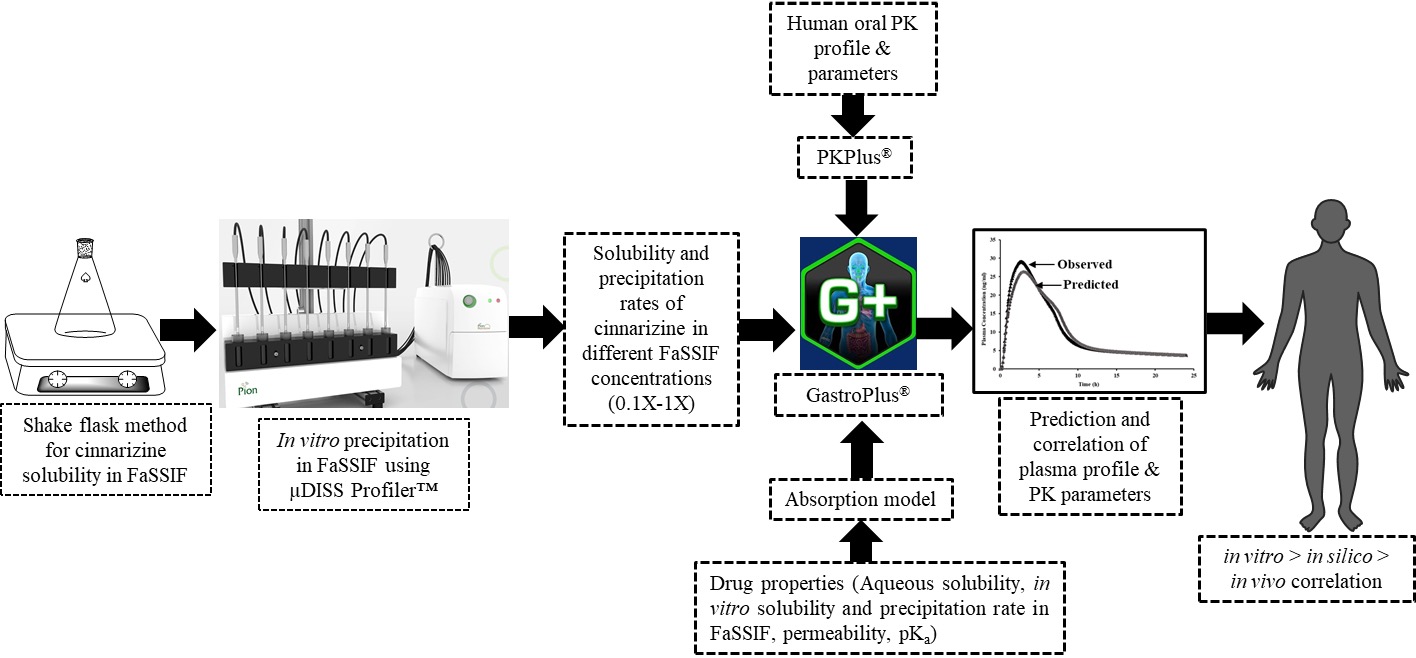
Purpose: Weakly basic compounds demonstrate pH-dependent solubility with high aqueous solubility in acidic pH-conditions, but precipitation is likely to occur in the intestine. Reports regarding the prediction methods for oral absorption of poorly soluble weak base compounds using physiologically based biopharmaceutics model and in vitro dissolution experiments have been published. The limitations of these methods include inadequate validation with human data and overestimation of precipitation. Thus, to avoid failure in the development of weak basic drugs, it is imperative to evaluate the oral absorption as early as possible. To this end, the aim of this study was to evaluate the impact of precipitation in different concentrations of fasted state simulates intestinal fluid (FaSSIF) as a surrogate for the in vivo variability in the endogenous bile salts concentration. We present herein an in vitro data modeling approach for cinnarizine, a weakly basic compound known to have poor solubility in the small intestine and precipitation behavior in vivo. A GastroPlus® model, enabling the introduction of solubility and precipitation rates obtained from in vitro investigations was developed.
Methods: The solubility at 4/24 h and precipitation rates of cinnarizine in different concentrations of biorelevant media (FaSSIF 0.1X, 0.2X, 0.5X, 1.0X) was measured using shake flask method at 37 ºC and µDISS Profiler™ (Pion Inc. Billerica, MA, USA) respectively. A GastroPlus® model enabling the introduction of solubility and precipitation rates obtained from in vitro investigations was included. The model included incorporation of CaCo2 permeability data. The performance of the approach was then evaluated by comparing the predicted plasma profiles, pharmacokinetic parameters, and oral absorption of cinnarizine with those reported from clinical studies.
Results: The results demonstrate that under fasted state conditions (FaSSIF 0.1X-1.0X), cinnarizine demonstrated a relationship between concentration of bile salt in FaSSIF and corresponding solubility and precipitation rates. The solubility results were in good agreement with the physicochemical properties of cinnarizine and with previously published solubility results in biorelevant media The plasma profiles were accurately predicted when a precipitation-integrated modeling approach was used for 25 mg immediate release tablet dosage for cinnarizine. The models developed here help to simulate plasma profiles of poorly soluble weak bases which tend to precipitate supporting the application of an integrated approach of incorporating in vitro precipitation kinetics to in vivo prediction.
Conclusion: It can be concluded that for poorly soluble weak base compounds with moderate permeability, precipitation during fasted state gastric emptying may play a critical role on oral drug absorption and thus on in vivo drug performance. The validated precipitation models developed for in vivo oral absorption simulations demonstrate the utility of in vitro experimental data to increase the confidence in computational oral absorption model predictions, thus supporting biopharmaceutics scientists evaluating the risk of in vivo precipitation impacting drug or drug product performance.
Acknowledgments: The authors would like to thank Ms. Aditi Panchal, Dr. David Harris and Dr. Marta Venczel for their inputs, assistance, and support to the work in this abstract.
Clinical Pharmacology - Chemical - Modeling and Simulation
Category: Late Breaking Poster Abstract
(T1530-11-62) Effect of Intestinal Precipitation on Cinnarizine Plasma Profile in Fasted State Employing Biorelevant In Vitro Tools and GastroPlus® Modeling
Tuesday, October 18, 2022
3:30 PM – 4:30 PM ET
- SK
Siddharth Kesharwani, Ph.D.
Sanofi
Waltham, Massachusetts, United States - SK
Siddharth Kesharwani, Ph.D.
Sanofi
Waltham, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Purpose: Weakly basic compounds demonstrate pH-dependent solubility with high aqueous solubility in acidic pH-conditions, but precipitation is likely to occur in the intestine. Reports regarding the prediction methods for oral absorption of poorly soluble weak base compounds using physiologically based biopharmaceutics model and in vitro dissolution experiments have been published. The limitations of these methods include inadequate validation with human data and overestimation of precipitation. Thus, to avoid failure in the development of weak basic drugs, it is imperative to evaluate the oral absorption as early as possible. To this end, the aim of this study was to evaluate the impact of precipitation in different concentrations of fasted state simulates intestinal fluid (FaSSIF) as a surrogate for the in vivo variability in the endogenous bile salts concentration. We present herein an in vitro data modeling approach for cinnarizine, a weakly basic compound known to have poor solubility in the small intestine and precipitation behavior in vivo. A GastroPlus® model, enabling the introduction of solubility and precipitation rates obtained from in vitro investigations was developed.
Methods: The solubility at 4/24 h and precipitation rates of cinnarizine in different concentrations of biorelevant media (FaSSIF 0.1X, 0.2X, 0.5X, 1.0X) was measured using shake flask method at 37 ºC and µDISS Profiler™ (Pion Inc. Billerica, MA, USA) respectively. A GastroPlus® model enabling the introduction of solubility and precipitation rates obtained from in vitro investigations was included. The model included incorporation of CaCo2 permeability data. The performance of the approach was then evaluated by comparing the predicted plasma profiles, pharmacokinetic parameters, and oral absorption of cinnarizine with those reported from clinical studies.
Results: The results demonstrate that under fasted state conditions (FaSSIF 0.1X-1.0X), cinnarizine demonstrated a relationship between concentration of bile salt in FaSSIF and corresponding solubility and precipitation rates. The solubility results were in good agreement with the physicochemical properties of cinnarizine and with previously published solubility results in biorelevant media The plasma profiles were accurately predicted when a precipitation-integrated modeling approach was used for 25 mg immediate release tablet dosage for cinnarizine. The models developed here help to simulate plasma profiles of poorly soluble weak bases which tend to precipitate supporting the application of an integrated approach of incorporating in vitro precipitation kinetics to in vivo prediction.
Conclusion: It can be concluded that for poorly soluble weak base compounds with moderate permeability, precipitation during fasted state gastric emptying may play a critical role on oral drug absorption and thus on in vivo drug performance. The validated precipitation models developed for in vivo oral absorption simulations demonstrate the utility of in vitro experimental data to increase the confidence in computational oral absorption model predictions, thus supporting biopharmaceutics scientists evaluating the risk of in vivo precipitation impacting drug or drug product performance.
Acknowledgments: The authors would like to thank Ms. Aditi Panchal, Dr. David Harris and Dr. Marta Venczel for their inputs, assistance, and support to the work in this abstract.

