Back
Purpose: The current understanding of dsRNA’s function is limited, which is typically associated with viral infection, immune regulations and damage-associated regeneration within eukaryotic cells. A recent report identified a novel class of dsRNA-glycan conjugates, glycoRNAs, from the living cell membrane, which indicates that it may play a role in signaling. More specifically, the membrane-bound dsRNA may serve as a new functional scaffold for aberrant glycosylation related to congenital disorders of glycosylation related to proteins and lipids. The dsRNAs may also engage in the initiation of signaling pathways such as Toll-Like Receptor 3 (TLR3), related to promoting regeneration of hair follicles in hair follicle stem cells. By studying the expression level of membrane-bound dsRNA and their function in the progenitor cell lines like HEI-OC1 cell, it is highly desirable to discover a new mechanism of pathways related to cell regeneration and specialization, which is critically needed for understanding cellular regenerative signaling pathways and developing novel regenerative therapy.
Methods: We utilized flow cytometry to compare and quantify the expression level of membrane-bound dsRNAs on cell lines with different regenerative potentials such as progenitor cell line HEI-OC1, primary cells isolated from healthy pancreas and tumor cell lines like HeLa. In the flow analysis, anti-dsRNA J2 monoclonal antibody incubated with 200K live cells at 4°C for 30min followed by the incubation with IRDye680 labeled secondary antibody (2° Ab) under the same condition. The cells were then submitted to CytoFlex and the data were then analyzed by FlowJo. We then utilized HEI-OC1 cell line with high dsRNA level on the cell surface as the in vitro model to obtain and investigate the sequences of dsRNA and its population. We utilized standard RNA extraction methods with QIAzol to isolate total RNAs and dsRNA from the whole cells, plasma membrane, and cytoplasm. The resulting RNAs were then quantified and analyzed with Bioanalyzer 2100.
Results: As shown in the Scheme in Fig.1, we examined dsRNA expression level on different cells with flow cytometry, obtained dsRNA sequences from different cellular compartments and the abundance by RNA-seq, and finally will map out the membrane-bound dsRNA atlas to the genome to predict the potential function of the dsRNA. In Fig. 2A, the cartoon model shows the binding prediction of anti-dsRNA J2 monoclonal antibody against dsRNA on the cell surface. After binding of J2 Ab and surface dsRNA, secondary antibody modified with IRDye680RD recognizes J2 antibody and the signal can be detected with flow cytometer. The flow data show that HEI-OC1 cell line, a progenitor cell line with differentiative ability, present great amount of dsRNA on the cell surface. We then asked that if the dsRNA presented on the HEI-OC1 cell surface can be enriched and obtained for sequencing analysis. As shown in figure 3A, we firstly isolated crude plasma membrane with homogenization and centrifugation and the flow analysis showed that the resulting plasma membrane can efficiently bind with J2 modified beads. It is shown in Fig 3B that total RNA and dsRNA isolated from different subcellular compartments had relatively good quality for downstream sequencing analysis.
Conclusion: In this study, dsRNAs were found on cell membranes from the HEI-OC1 cell line, a progenitor cell line with good differentiative ability as reported by Kalinec et al. by using anti-dsRNA antibody and flow cytometry. The binding between J2 modified beads and plasma membrane labeled with amine-activated dye detected by flow cytometry suggested the membrane-bound RNA integrity and its presence. RNA from the whole cells, cytoplasma, and plasma membrane and were extracted and processed for further sequencing and deep bioinformatic analysis. In short, we discovered new dsRNA on HEI-OC1 cell surface and isolated membrane-bound dsRNA for the downstream sequencing that can be further utilized for the in-depth understanding of dsRNA on the cell membrane.
References: 1. Cell 184, 3109–3124 (2021).
2. Cell 184(12):3080-3081 (2021).
3. Nat Commun 12, 1582 (2021).
4. Nat Rev Mol Cell Biol 21, 585–606 (2020).
5. Cell Stem Cell 17, 139–151 (2015).
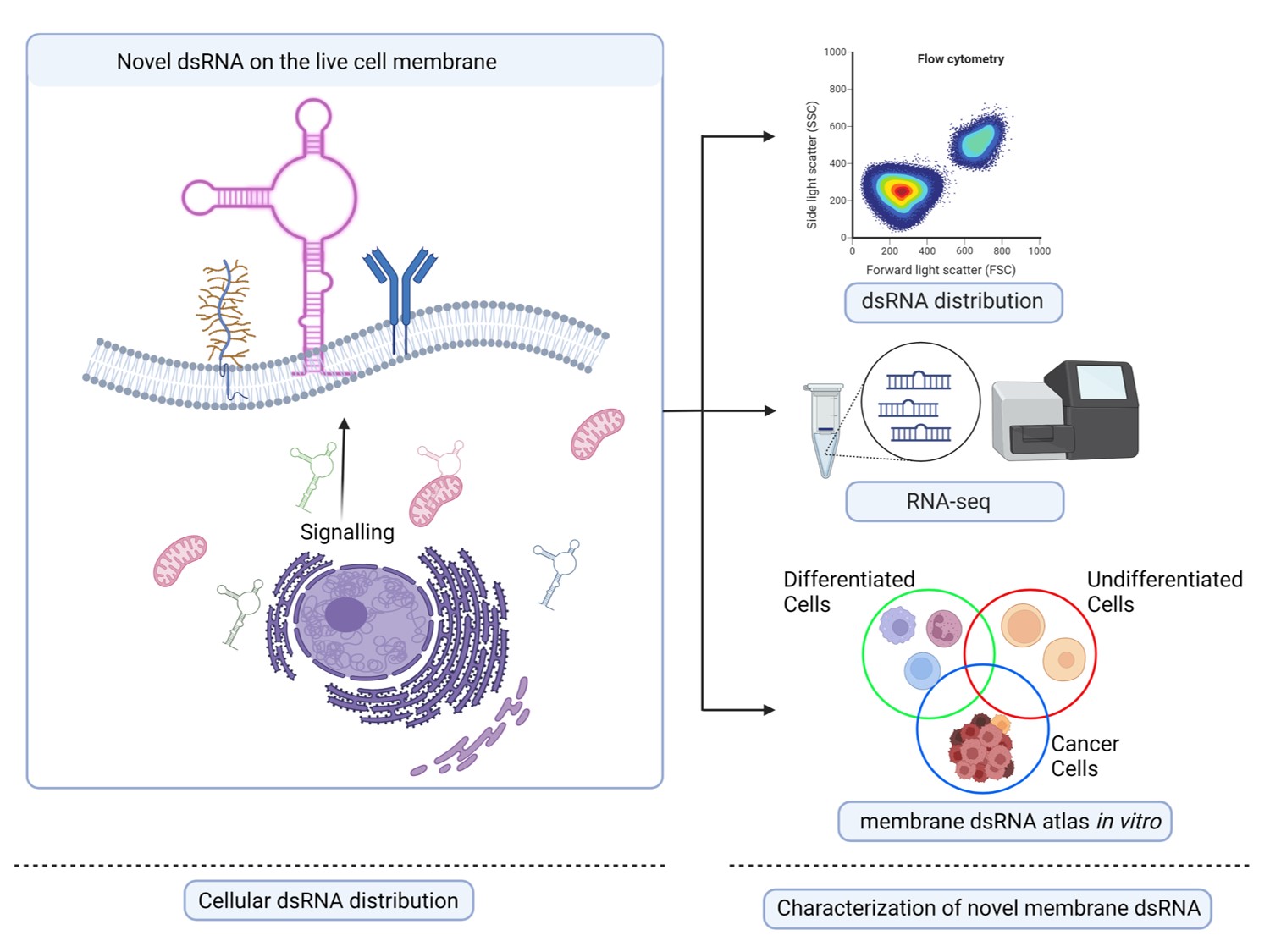
Figure 1: Schematic illustration of the investigation of dsRNA isolated from cytoplasm and plasma membrane of live cells
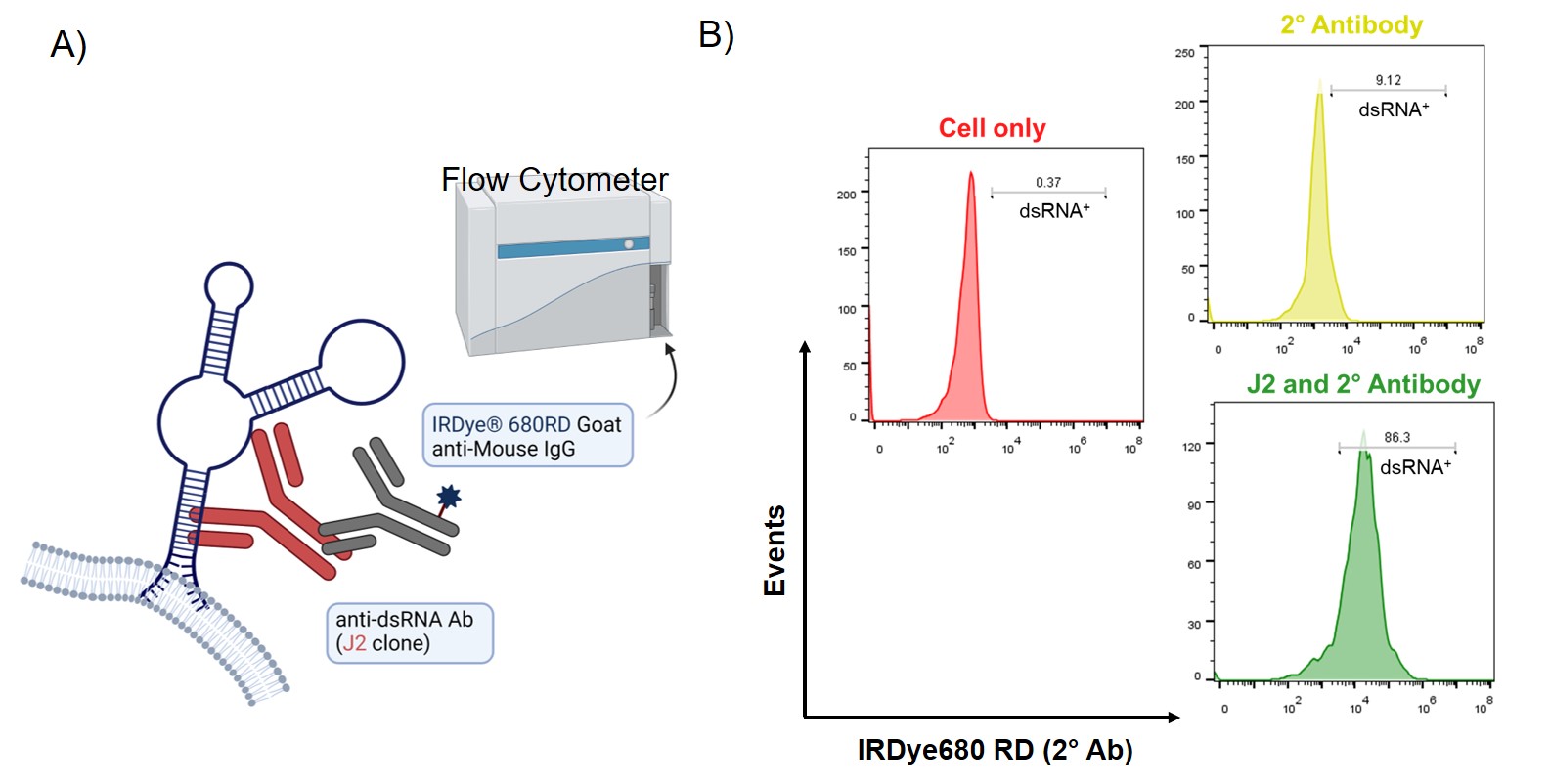
Figure 2: A) Schematic illustration of the binding between the anti-dsRNA antibody (J2) and membrane-bound dsRNA on the cell surface. B) Flow analysis of HEI-OC1 cells stained with the J2 antibody. Gated region represents high J2 binding. The experiments were conducted in duplicates.
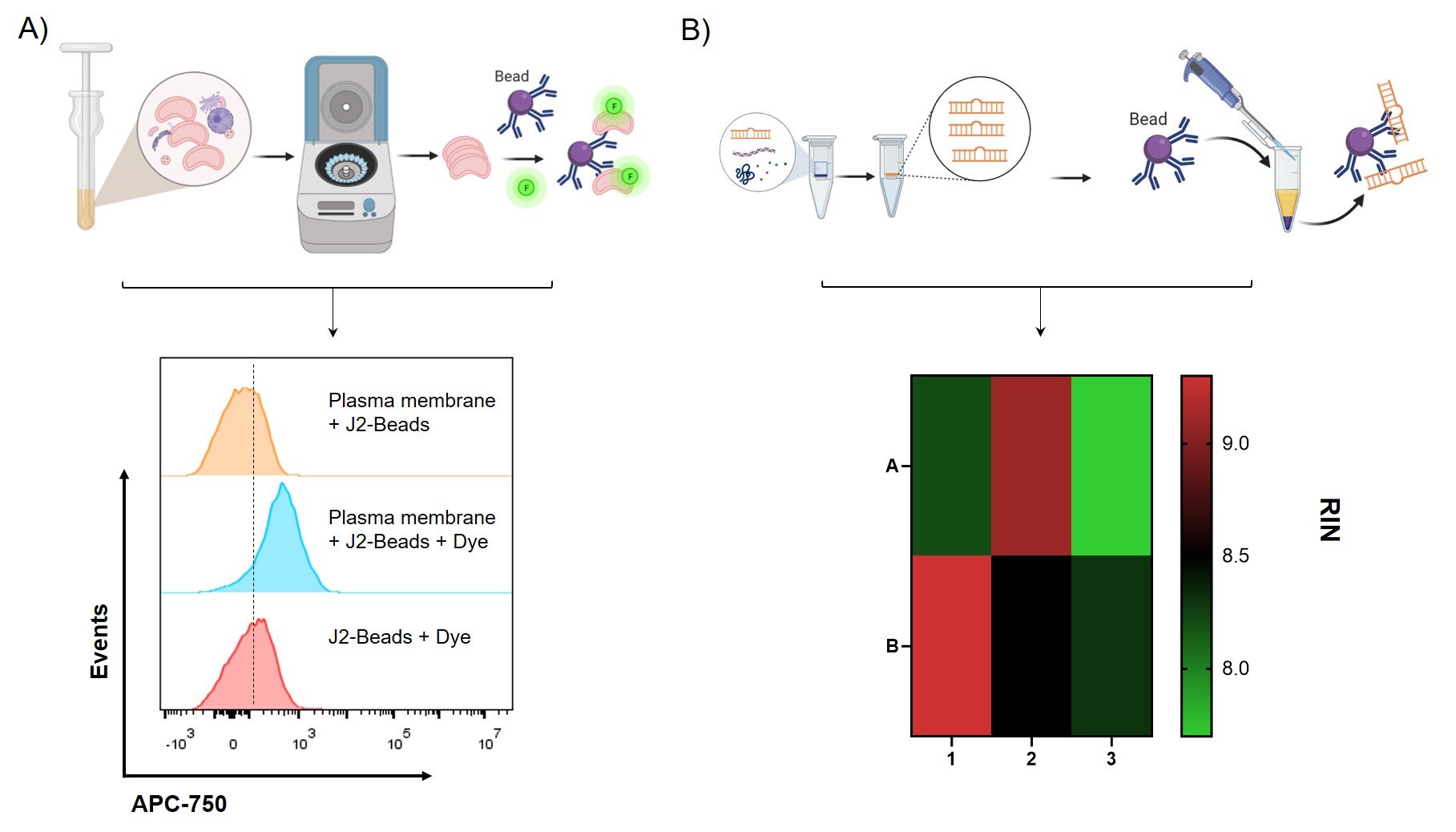
Figure 3: A) Schematic illustration of purification and analysis of plasma membrane immobilized on the anti-dsRNA J2 antibody modified Dynabeads. The resulting flow histogram of plasma membrane captured by J2-beads analyzed by FlowJo. The plasma membrane was labeled with dye that react with amine groups on the membrane. B) Schematic illustration of RNA extraction process and the resulting RNA quality (RNA integrity number by Eukaryote Total RNA PICO Bioanalyzer 2100) of dsRNA before and after J2 immunoprecipitation followed by the schematic protocol. 1-3 represents whole cell, plasma membrane, cytoplasm respectively. A is after J2 immunoprecipitation, representing dsRNA and B is before J2 immunoprecipitation, total RNA.
Category: Poster Abstract
(T1230-10-60) Membrane-Bound Double-Stranded RNAs as a Potential Biomarker for Regenerative Therapy
Tuesday, October 18, 2022
12:30 PM – 1:30 PM ET
- RH
Rachell Hawkes
University of Florida
Gainesville, Florida, United States - RH
Rachell Hawkes
University of Florida
Gainesville, Florida, United States
Presenting Author(s)
Main Author(s)
Purpose: The current understanding of dsRNA’s function is limited, which is typically associated with viral infection, immune regulations and damage-associated regeneration within eukaryotic cells. A recent report identified a novel class of dsRNA-glycan conjugates, glycoRNAs, from the living cell membrane, which indicates that it may play a role in signaling. More specifically, the membrane-bound dsRNA may serve as a new functional scaffold for aberrant glycosylation related to congenital disorders of glycosylation related to proteins and lipids. The dsRNAs may also engage in the initiation of signaling pathways such as Toll-Like Receptor 3 (TLR3), related to promoting regeneration of hair follicles in hair follicle stem cells. By studying the expression level of membrane-bound dsRNA and their function in the progenitor cell lines like HEI-OC1 cell, it is highly desirable to discover a new mechanism of pathways related to cell regeneration and specialization, which is critically needed for understanding cellular regenerative signaling pathways and developing novel regenerative therapy.
Methods: We utilized flow cytometry to compare and quantify the expression level of membrane-bound dsRNAs on cell lines with different regenerative potentials such as progenitor cell line HEI-OC1, primary cells isolated from healthy pancreas and tumor cell lines like HeLa. In the flow analysis, anti-dsRNA J2 monoclonal antibody incubated with 200K live cells at 4°C for 30min followed by the incubation with IRDye680 labeled secondary antibody (2° Ab) under the same condition. The cells were then submitted to CytoFlex and the data were then analyzed by FlowJo. We then utilized HEI-OC1 cell line with high dsRNA level on the cell surface as the in vitro model to obtain and investigate the sequences of dsRNA and its population. We utilized standard RNA extraction methods with QIAzol to isolate total RNAs and dsRNA from the whole cells, plasma membrane, and cytoplasm. The resulting RNAs were then quantified and analyzed with Bioanalyzer 2100.
Results: As shown in the Scheme in Fig.1, we examined dsRNA expression level on different cells with flow cytometry, obtained dsRNA sequences from different cellular compartments and the abundance by RNA-seq, and finally will map out the membrane-bound dsRNA atlas to the genome to predict the potential function of the dsRNA. In Fig. 2A, the cartoon model shows the binding prediction of anti-dsRNA J2 monoclonal antibody against dsRNA on the cell surface. After binding of J2 Ab and surface dsRNA, secondary antibody modified with IRDye680RD recognizes J2 antibody and the signal can be detected with flow cytometer. The flow data show that HEI-OC1 cell line, a progenitor cell line with differentiative ability, present great amount of dsRNA on the cell surface. We then asked that if the dsRNA presented on the HEI-OC1 cell surface can be enriched and obtained for sequencing analysis. As shown in figure 3A, we firstly isolated crude plasma membrane with homogenization and centrifugation and the flow analysis showed that the resulting plasma membrane can efficiently bind with J2 modified beads. It is shown in Fig 3B that total RNA and dsRNA isolated from different subcellular compartments had relatively good quality for downstream sequencing analysis.
Conclusion: In this study, dsRNAs were found on cell membranes from the HEI-OC1 cell line, a progenitor cell line with good differentiative ability as reported by Kalinec et al. by using anti-dsRNA antibody and flow cytometry. The binding between J2 modified beads and plasma membrane labeled with amine-activated dye detected by flow cytometry suggested the membrane-bound RNA integrity and its presence. RNA from the whole cells, cytoplasma, and plasma membrane and were extracted and processed for further sequencing and deep bioinformatic analysis. In short, we discovered new dsRNA on HEI-OC1 cell surface and isolated membrane-bound dsRNA for the downstream sequencing that can be further utilized for the in-depth understanding of dsRNA on the cell membrane.
References: 1. Cell 184, 3109–3124 (2021).
2. Cell 184(12):3080-3081 (2021).
3. Nat Commun 12, 1582 (2021).
4. Nat Rev Mol Cell Biol 21, 585–606 (2020).
5. Cell Stem Cell 17, 139–151 (2015).

Figure 1: Schematic illustration of the investigation of dsRNA isolated from cytoplasm and plasma membrane of live cells

Figure 2: A) Schematic illustration of the binding between the anti-dsRNA antibody (J2) and membrane-bound dsRNA on the cell surface. B) Flow analysis of HEI-OC1 cells stained with the J2 antibody. Gated region represents high J2 binding. The experiments were conducted in duplicates.

Figure 3: A) Schematic illustration of purification and analysis of plasma membrane immobilized on the anti-dsRNA J2 antibody modified Dynabeads. The resulting flow histogram of plasma membrane captured by J2-beads analyzed by FlowJo. The plasma membrane was labeled with dye that react with amine groups on the membrane. B) Schematic illustration of RNA extraction process and the resulting RNA quality (RNA integrity number by Eukaryote Total RNA PICO Bioanalyzer 2100) of dsRNA before and after J2 immunoprecipitation followed by the schematic protocol. 1-3 represents whole cell, plasma membrane, cytoplasm respectively. A is after J2 immunoprecipitation, representing dsRNA and B is before J2 immunoprecipitation, total RNA.
