Back
Purpose: Human organic anion transporter 4 (OAT4) is mainly expressed in the kidney and the placenta. It plays important roles in the distribution and elimination of numerous endogenous metabolites, environmental toxins, and medications in the body. Post-translational modifications (PTMs) of OAT4 were reported to regulate its transport activity and protein expression. The purpose of this study is to explore the regulatory effects of insulin-like growth factor 1 (IGF-1) and potential downstream signaling pathways on one of the significant PTMs (SUMOylation), protein expression, and transport activity of OAT4.
Methods: OAT4-expressing COS-7 cells were treated with IGF-1 and ipatasertib, a PKB-specific inhibitor and anti-cancer drug under clinical trials. Treated cells were then examined for transport activity (via cell uptake assay), protein expression (via biotinylation assay and western blotting), and SUMOylation (via immunoprecipitation and western blotting) of OAT4.
Results: IGF-1 treatment increased OAT4 uptake activity in a dose-dependent manner, with 15 to 40% of increase after 1 nM to 100 nM of IGF-1 treatment. This stimulatory effect from IGF-1 was reversed by treatment of the PKB-specific inhibitor, ipatasertib. Furthermore, the increase of OAT4 transport activity by IGF-1 was accompanied by the increases in cell surface expression (~30%) and SUMOylation (~50%) of the transporter. Again, ipatasertib was able to reverse both the increases of OAT4 surface expression and SUMOylation, indicating PKB specificity of this regulation by IGF-1.
Conclusion: Our results demonstrated that IGF-1 regulates the SUMOylation, protein expression at cell surface, and transport activity of OAT4 through PKB signaling pathway. Fluctuating levels of IGF-1 in the body were observed under various physiological and pathological conditions, which could affect OAT4-mediated disposition of drugs and environmental toxins. Our study revealed valuable information on the potential mechanism underlying the regulation of OAT4 by IGF-1.
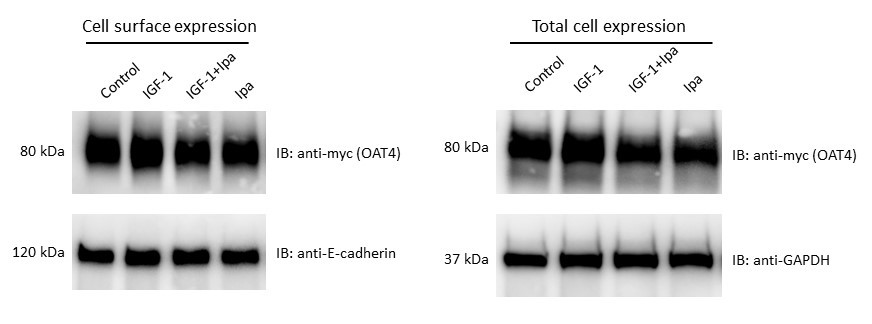
Fig. 3. Effect of IGF-1 on OAT4 protein expression. (a). Cell surface expression of hOAT4. hOAT4-expressing COS-7 cells were treated with IGF-1 (100 nM, 2h). Cells were labeled with biotinylation reagent. Biotinylated cell surface proteins were purified with streptavidin resin, followed by immunoblotting (IB) with anti-myc antibody. Epitope myc was tagged to OAT4 for immune-detection. Anti-E-cadherin was used as a loading control for proteins on the cell surface. (b). Total cell expression of OAT4. hOAT4-expressing COS-7 cells were treated with IGF-1 (100 nM, 2h). Cells were then lysed, followed by immunoblotting (IB) with anti-Myc antibody. Anti-GAPDH was used as a loading control for total proteins.
.jpg)
Fig. 1. IGF-1 increased OAT4 transport activity in a dose-dependent manner. hOAT4-expressing cells were treated with 1-100 nM IGF-1 for 2 hr, followed by [3H] estrone sulfate uptake assay. Uptake activity was expressed as a percentage of the uptake values measured in control cells. The data represent uptake into hOAT4-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD (n = 3). *P <0.05.
.jpg)
Fig. 2. IGF-1-stimulated OAT4 transport activity was blocked by a clinical therapeutic. hOAT4-expressing cells were treated with IGF-1 (100 nM, 2h) in the presence and absence of PKB inhibitor Ipatasertib (Ipa) (10 μM) , followed by [3H] estrone sulfate uptake assay. Uptake activity was expressed as a percentage of the uptake values measured in control cells. The data represent uptake into hOAT4-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD (n = 3). *P <0.05.
Discovery and Basic Research - Biology
Category: Late Breaking Poster Abstract
(T1230-10-55) Insulin-Like Growth Factor 1 Modulates Transport Activity, Protein Expression, and SUMOylation of Human Organic Anion Transporter 4 through Protein Kinase B Signaling Pathway
Tuesday, October 18, 2022
12:30 PM – 1:30 PM ET
- ZY
Zhou Yu, MS
Rutgers University
Piscataway, New Jersey, United States - ZY
Zhou Yu, MS
Rutgers University
Piscataway, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: Human organic anion transporter 4 (OAT4) is mainly expressed in the kidney and the placenta. It plays important roles in the distribution and elimination of numerous endogenous metabolites, environmental toxins, and medications in the body. Post-translational modifications (PTMs) of OAT4 were reported to regulate its transport activity and protein expression. The purpose of this study is to explore the regulatory effects of insulin-like growth factor 1 (IGF-1) and potential downstream signaling pathways on one of the significant PTMs (SUMOylation), protein expression, and transport activity of OAT4.
Methods: OAT4-expressing COS-7 cells were treated with IGF-1 and ipatasertib, a PKB-specific inhibitor and anti-cancer drug under clinical trials. Treated cells were then examined for transport activity (via cell uptake assay), protein expression (via biotinylation assay and western blotting), and SUMOylation (via immunoprecipitation and western blotting) of OAT4.
Results: IGF-1 treatment increased OAT4 uptake activity in a dose-dependent manner, with 15 to 40% of increase after 1 nM to 100 nM of IGF-1 treatment. This stimulatory effect from IGF-1 was reversed by treatment of the PKB-specific inhibitor, ipatasertib. Furthermore, the increase of OAT4 transport activity by IGF-1 was accompanied by the increases in cell surface expression (~30%) and SUMOylation (~50%) of the transporter. Again, ipatasertib was able to reverse both the increases of OAT4 surface expression and SUMOylation, indicating PKB specificity of this regulation by IGF-1.
Conclusion: Our results demonstrated that IGF-1 regulates the SUMOylation, protein expression at cell surface, and transport activity of OAT4 through PKB signaling pathway. Fluctuating levels of IGF-1 in the body were observed under various physiological and pathological conditions, which could affect OAT4-mediated disposition of drugs and environmental toxins. Our study revealed valuable information on the potential mechanism underlying the regulation of OAT4 by IGF-1.

Fig. 3. Effect of IGF-1 on OAT4 protein expression. (a). Cell surface expression of hOAT4. hOAT4-expressing COS-7 cells were treated with IGF-1 (100 nM, 2h). Cells were labeled with biotinylation reagent. Biotinylated cell surface proteins were purified with streptavidin resin, followed by immunoblotting (IB) with anti-myc antibody. Epitope myc was tagged to OAT4 for immune-detection. Anti-E-cadherin was used as a loading control for proteins on the cell surface. (b). Total cell expression of OAT4. hOAT4-expressing COS-7 cells were treated with IGF-1 (100 nM, 2h). Cells were then lysed, followed by immunoblotting (IB) with anti-Myc antibody. Anti-GAPDH was used as a loading control for total proteins.
.jpg)
Fig. 1. IGF-1 increased OAT4 transport activity in a dose-dependent manner. hOAT4-expressing cells were treated with 1-100 nM IGF-1 for 2 hr, followed by [3H] estrone sulfate uptake assay. Uptake activity was expressed as a percentage of the uptake values measured in control cells. The data represent uptake into hOAT4-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD (n = 3). *P <0.05.
.jpg)
Fig. 2. IGF-1-stimulated OAT4 transport activity was blocked by a clinical therapeutic. hOAT4-expressing cells were treated with IGF-1 (100 nM, 2h) in the presence and absence of PKB inhibitor Ipatasertib (Ipa) (10 μM) , followed by [3H] estrone sulfate uptake assay. Uptake activity was expressed as a percentage of the uptake values measured in control cells. The data represent uptake into hOAT4-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD (n = 3). *P <0.05.
