Back
Purpose: Solubility enhancement through formulation for intravenous (IV) administration is problematic issue for poorly water-soluble drugs in preclinical development. Nanoparticles (NPs) fabricated from an FDA-approved polymer, polylactic-co-glycolic acid (PLGA), has been explored in recent years for alternative preparation of a commercial formulation to the solvent-based drug solubilization approach. However, synthesis of PLGA NPs through various techniques, such as solvent evaporation or diffusion methods, always is compromised by particle aggregation, nonuniform particles size, low payload, and batch-to-batch inconsistencies. Here, the objective of this study was to explore the development of a PLGA NPs drug delivery system as suitable IV formulations for translational studies.
Methods: Solubility enhancement through formulation for intravenous (IV) administration is problematic issue for poorly water-soluble drugs in preclinical development. Nanoparticles (NPs) fabricated from an FDA-approved polymer, polylactic-co-glycolic acid (PLGA), has been explored in recent years for alternative preparation of a commercial formulation to the solvent-based drug solubilization approach. However, synthesis of PLGA NPs through various techniques, such as solvent evaporation or diffusion methods, always is compromised by particle aggregation, nonuniform particles size, low payload, and batch-to-batch inconsistencies. Here, the objective of this study was to explore the development of a PLGA NPs drug delivery system as suitable IV formulations for translational studies.
Results: An understanding of the critical processing parameters like PLGA concentration, solvent fraction, and pressure during synthesis used in putting forward the critical quality attributes such as size (133 to 164 nm), charge (-6 to -15.8 mV), encapsulation efficiency (40% to 52%), providing an effective control strategy in development of tunable NP libraries rather than a single formulation. Colloidal dispersions of PLGA after evaporation or lyophilization are very stable during short-term storage at different conditions. Further evaluation of drug delivery and safety by PLGA NPs through IV route was conducted in single jugular vein cannulated rats at a single dose of 2.5 mg/kg. Rats were carefully monitored, showing no side effects. The decreased exposure and shorter half time indicated a rapid clearance of PLGA NPs by cells of the mononuclear phagocytic system (MPS) in comparison with other cosolvent formulation.
Conclusion: Emulsification-evaporation with DCM coupled to high pressure homogenization is a reliable procedure for the reproducible and refined synthesis of PLGA nanoparticles for intravenous drug delivery.
References: Ferrari R, Sponchioni M, Morbidelli M, Moscatelli D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018 Dec 13;10(48):22701-22719
Chen Y, Gao XQ, Gupta R, Ma J et al. Development and Validation of a LC-MS/MS Method for AC1LPSZG and Pharmacokinetics Application in Rats.J Chromatogr Sci. 2022 Jan 1;60(1):26-34
Acknowledgments: Study was funded by Cancer Prevention & Research Institute of Texas Core Facilities Support Awards (RP180748) and NIH’s Research Centers in Minority Institutes Program (RCMI, U54MD007605).
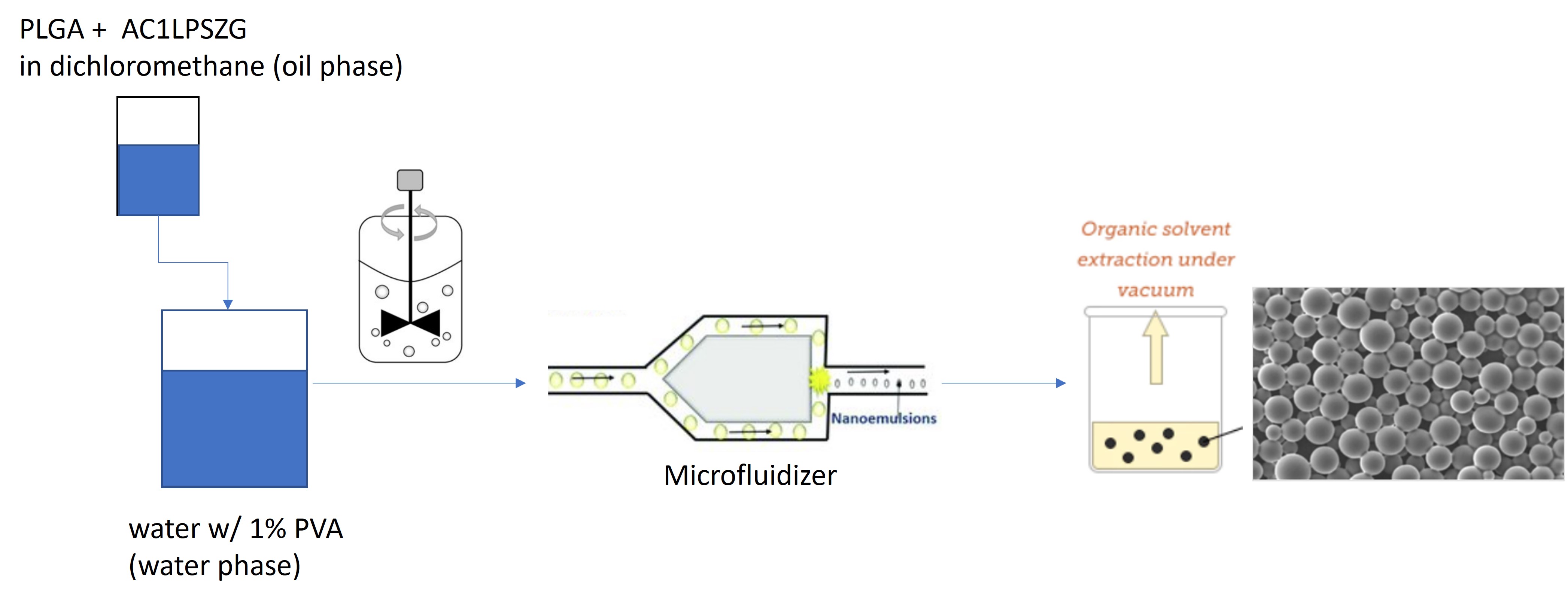
Preparation of PLGA nanoparticles as drug delivery system by microfluidizer (high pressure homogenizer)
.jpg)
Parameters and associated levels used in DOE approach
.jpg)
Stability and pharmacokinetic profile of PLGA NPs as drug delivery system for AC1LPSZG
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1030-04-21) Practical Preparation of Reproducible PLGA Nanoparticle for Intravenous Drug Administration
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- YC
Yuan Chen, Ph.D.
Texas Southern University
Houston, Texas, United States - YC
Yuan Chen, Ph.D.
Texas Southern University
Houston, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: Solubility enhancement through formulation for intravenous (IV) administration is problematic issue for poorly water-soluble drugs in preclinical development. Nanoparticles (NPs) fabricated from an FDA-approved polymer, polylactic-co-glycolic acid (PLGA), has been explored in recent years for alternative preparation of a commercial formulation to the solvent-based drug solubilization approach. However, synthesis of PLGA NPs through various techniques, such as solvent evaporation or diffusion methods, always is compromised by particle aggregation, nonuniform particles size, low payload, and batch-to-batch inconsistencies. Here, the objective of this study was to explore the development of a PLGA NPs drug delivery system as suitable IV formulations for translational studies.
Methods: Solubility enhancement through formulation for intravenous (IV) administration is problematic issue for poorly water-soluble drugs in preclinical development. Nanoparticles (NPs) fabricated from an FDA-approved polymer, polylactic-co-glycolic acid (PLGA), has been explored in recent years for alternative preparation of a commercial formulation to the solvent-based drug solubilization approach. However, synthesis of PLGA NPs through various techniques, such as solvent evaporation or diffusion methods, always is compromised by particle aggregation, nonuniform particles size, low payload, and batch-to-batch inconsistencies. Here, the objective of this study was to explore the development of a PLGA NPs drug delivery system as suitable IV formulations for translational studies.
Results: An understanding of the critical processing parameters like PLGA concentration, solvent fraction, and pressure during synthesis used in putting forward the critical quality attributes such as size (133 to 164 nm), charge (-6 to -15.8 mV), encapsulation efficiency (40% to 52%), providing an effective control strategy in development of tunable NP libraries rather than a single formulation. Colloidal dispersions of PLGA after evaporation or lyophilization are very stable during short-term storage at different conditions. Further evaluation of drug delivery and safety by PLGA NPs through IV route was conducted in single jugular vein cannulated rats at a single dose of 2.5 mg/kg. Rats were carefully monitored, showing no side effects. The decreased exposure and shorter half time indicated a rapid clearance of PLGA NPs by cells of the mononuclear phagocytic system (MPS) in comparison with other cosolvent formulation.
Conclusion: Emulsification-evaporation with DCM coupled to high pressure homogenization is a reliable procedure for the reproducible and refined synthesis of PLGA nanoparticles for intravenous drug delivery.
References: Ferrari R, Sponchioni M, Morbidelli M, Moscatelli D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018 Dec 13;10(48):22701-22719
Chen Y, Gao XQ, Gupta R, Ma J et al. Development and Validation of a LC-MS/MS Method for AC1LPSZG and Pharmacokinetics Application in Rats.J Chromatogr Sci. 2022 Jan 1;60(1):26-34
Acknowledgments: Study was funded by Cancer Prevention & Research Institute of Texas Core Facilities Support Awards (RP180748) and NIH’s Research Centers in Minority Institutes Program (RCMI, U54MD007605).

Preparation of PLGA nanoparticles as drug delivery system by microfluidizer (high pressure homogenizer)
.jpg)
Parameters and associated levels used in DOE approach
.jpg)
Stability and pharmacokinetic profile of PLGA NPs as drug delivery system for AC1LPSZG
