Back
Purpose: Proteasome inhibitors (PIs), currently approved for hematologic malignancies, work by primarily inhibiting the b5 active site of the proteasome, resulting in a partial inhibition of misfolded protein breakdown. Because cancer cells produce higher levels of misfolded proteins, a buildup of these proteins without a functioning proteasome leads cancer cells to undergo apoptosis. We want to expand this therapeutic approach to solid tumors and are focusing on triple-negative breast cancer (TNBC), which lacks targeted treatment and has the lowest survival rates among breast cancers. TNBCs are addicted to the proteasome but the mechanistic reason for this dependency is not known. Sensitivity of tumor cells to proteasome inhibitors depends on the load (i.e., total amount of proteins degraded) on the proteasome. Majority of proteasome substrates are misfolded nascent polypeptides. Cells usually respond to the accumulation of misfolded proteins by reducing protein synthesis through the phosphorylation of translation initiation factor eIF2a. In theory, reducing protein synthesis should lead to the reduction of proteasome load, however, we surprisingly found a decrease in eIF2a phosphorylation in PI-treated TNBC cells. We hypothesize that this phenomenon is responsible for the increased sensitivity of TNBC cells to proteasome inhibitors because it would not allow cells to reduce protein synthesis and load on proteasomes.
Methods: SUM149 and MDA-MB-231 TNBC cell lines were treated with Carfilzomib or Bortezomib, two FDA-approved proteasome inhibitors. Cells treated with Thapsigargin and Tunicamycin, known ER stress inducers, were used as positive controls. Western Blotting was performed to look changes in protein expression. SiRNA was used to knockdown the expression of CReP in TNBC cells.
Results: TNBC cells are sensitive to proteasome inhibitors because they up-regulate elF2α phosphatase. De-phosphorylation of elF2α promotes global protein synthesis, which further elevates the load on the proteasome, ultimately leading cells to undergo apoptosis.
Conclusion: TNBC cells are sensitive to proteasome inhibitors because they up-regulate eIF2α phosphatase. De-phosphorylation of eIF2α promotes global protein synthesis, which further elevates the load on the proteasome, ultimately leading cells to undergo apoptosis.
References: Weyburne, E. S. et al. Inhibition of the Proteasome β2 Site Sensitizes Triple-Negative Breast Cancer Cells to β5 Inhibitors and Suppresses Nrf1 Activation. Cell Chem. Biol. 24, 218–230 (2017).
Acknowledgements:R01 CA 213223
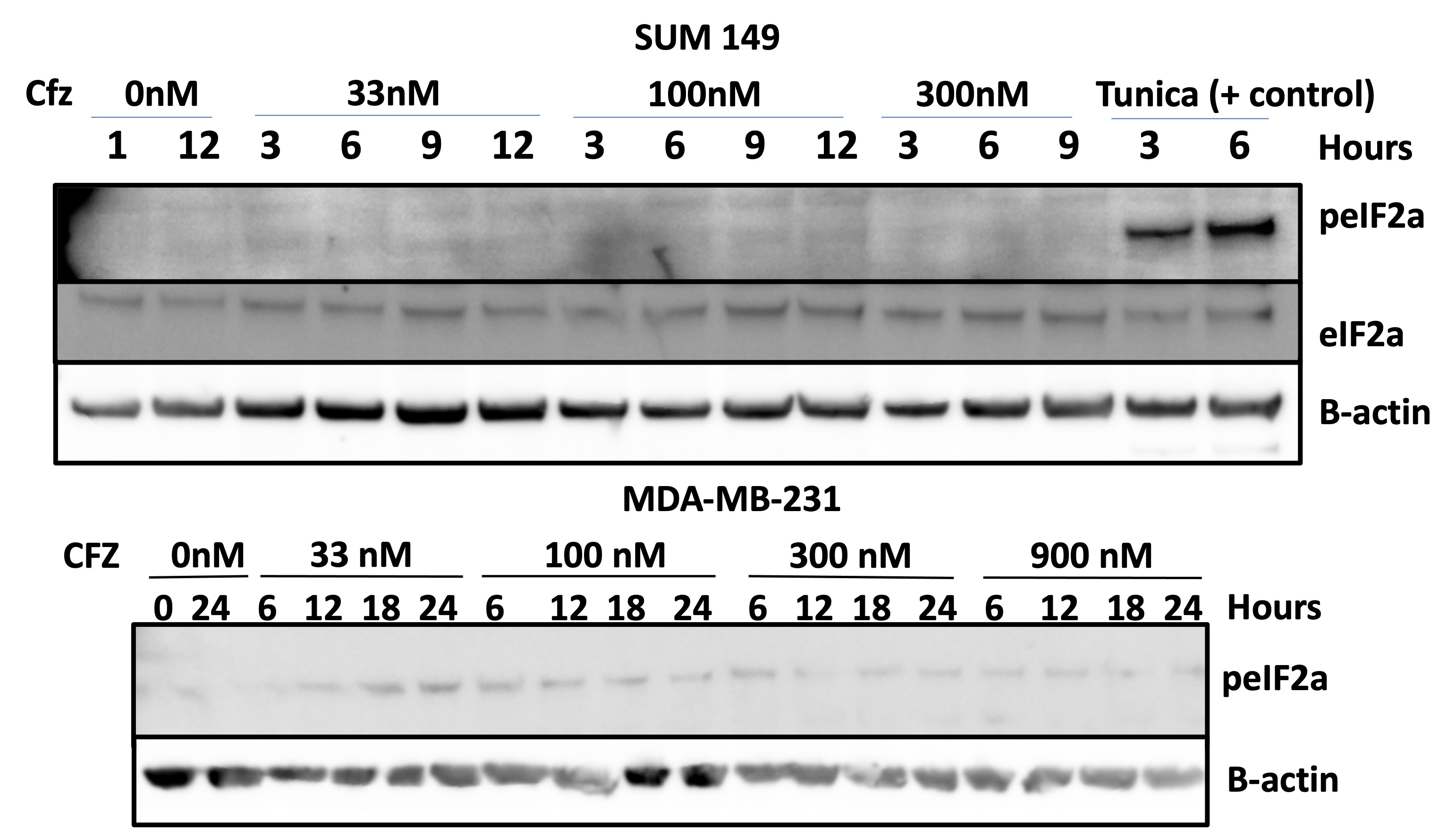
Lack of eIF2a phosphorylation observed in response to Carfilzomib in SUM149 TNBC cells. While an initial increase in eIF2a phosphorylation is observed at low concentrations in response to Carfilzomib in MDA-MB-231 TNBC cells, it decreases at higher concentrations and later time points.

eIF2a is dephosphorylated in response to Proteasome inhibitors, along with elevated CReP and GADD34, subunits of PP1C. P-eIF2a is restored when treated with Carfilzomib and Salubrinal, an inhibitor of eIF2a phosphatase PP1C, but continuous to has elevated CReP and GADD34 levels.

Inactivation of PP1C, using siRNA to knockdown CReP, blocks proteasome inhibitor-induced apoptosis.
Discovery and Basic Research - Pharmacology
Category: Poster Abstract
(W1130-05-29) Inactivation of Integrated Stress Response Pathway Sensitizes Triple-Negative Breast Cancer Cells to Proteasome Inhibitors
Wednesday, October 19, 2022
11:30 AM – 12:30 PM ET
- SS
Sriraja Srinivasa, BS
Auburn University
Auburn University, Alabama, United States - SS
Sriraja Srinivasa, BS
Auburn University
Auburn University, Alabama, United States
Presenting Author(s)
Main Author(s)
Purpose: Proteasome inhibitors (PIs), currently approved for hematologic malignancies, work by primarily inhibiting the b5 active site of the proteasome, resulting in a partial inhibition of misfolded protein breakdown. Because cancer cells produce higher levels of misfolded proteins, a buildup of these proteins without a functioning proteasome leads cancer cells to undergo apoptosis. We want to expand this therapeutic approach to solid tumors and are focusing on triple-negative breast cancer (TNBC), which lacks targeted treatment and has the lowest survival rates among breast cancers. TNBCs are addicted to the proteasome but the mechanistic reason for this dependency is not known. Sensitivity of tumor cells to proteasome inhibitors depends on the load (i.e., total amount of proteins degraded) on the proteasome. Majority of proteasome substrates are misfolded nascent polypeptides. Cells usually respond to the accumulation of misfolded proteins by reducing protein synthesis through the phosphorylation of translation initiation factor eIF2a. In theory, reducing protein synthesis should lead to the reduction of proteasome load, however, we surprisingly found a decrease in eIF2a phosphorylation in PI-treated TNBC cells. We hypothesize that this phenomenon is responsible for the increased sensitivity of TNBC cells to proteasome inhibitors because it would not allow cells to reduce protein synthesis and load on proteasomes.
Methods: SUM149 and MDA-MB-231 TNBC cell lines were treated with Carfilzomib or Bortezomib, two FDA-approved proteasome inhibitors. Cells treated with Thapsigargin and Tunicamycin, known ER stress inducers, were used as positive controls. Western Blotting was performed to look changes in protein expression. SiRNA was used to knockdown the expression of CReP in TNBC cells.
Results: TNBC cells are sensitive to proteasome inhibitors because they up-regulate elF2α phosphatase. De-phosphorylation of elF2α promotes global protein synthesis, which further elevates the load on the proteasome, ultimately leading cells to undergo apoptosis.
Conclusion: TNBC cells are sensitive to proteasome inhibitors because they up-regulate eIF2α phosphatase. De-phosphorylation of eIF2α promotes global protein synthesis, which further elevates the load on the proteasome, ultimately leading cells to undergo apoptosis.
References: Weyburne, E. S. et al. Inhibition of the Proteasome β2 Site Sensitizes Triple-Negative Breast Cancer Cells to β5 Inhibitors and Suppresses Nrf1 Activation. Cell Chem. Biol. 24, 218–230 (2017).
Acknowledgements:R01 CA 213223

Lack of eIF2a phosphorylation observed in response to Carfilzomib in SUM149 TNBC cells. While an initial increase in eIF2a phosphorylation is observed at low concentrations in response to Carfilzomib in MDA-MB-231 TNBC cells, it decreases at higher concentrations and later time points.

eIF2a is dephosphorylated in response to Proteasome inhibitors, along with elevated CReP and GADD34, subunits of PP1C. P-eIF2a is restored when treated with Carfilzomib and Salubrinal, an inhibitor of eIF2a phosphatase PP1C, but continuous to has elevated CReP and GADD34 levels.

Inactivation of PP1C, using siRNA to knockdown CReP, blocks proteasome inhibitor-induced apoptosis.
