Back
Purpose: Zika is a zoonotic disease caused by the Zika virus and transmitted to humans through mosquito bites. Researchers have observed a causal relationship between Zika infection and congenital birth defects in children born to infected mothers. Zika infection can also result in Guillain-Barr Syndrome, an autoimmune disorder affecting the peripheral nervous system. Unfortunately, there is a lack of an approved vaccine against Zika to date. Current vaccine modalities under research are administered via either subcutaneous or intramuscular injection. These are painful and reduce patient compliance. Moreover, Zika is endemic in developing countries where the distribution of vaccines is challenging due to the scarcity of cold-chain storage resources and trained personnel. Formulating a vaccine in dry powder form would enhance the stability and eliminate the need for cold-chain storage. Therefore, in this study, we have formulated Zika vaccine microparticles (MPs)-loaded dissolving microneedles (MNs) to provide a pain-free immunization alternative to conventional vaccines. We evaluated the efficacy of this vaccine in a preclinical mouse model by measuring antibody titers and cellular immune response.
Methods: The inactivated Zika virus-loaded vaccine MPs were formulated by a double emulsion solvent evaporation method followed by lyophilization using poly(lactic-co-glycolic) acid (PLGA) as the polymer. Adjuvant MPs encapsulating a combination of Alhydrogel® and MPL-A® were formulated following a similar method. After testing for size, charge, morphology, encapsulation efficiency, and in vitro release, the vaccine MPs with or without adjuvant were loaded into dissolving MNs. The average needle length of MNs was measured using scanning electron microscopy (SEM). The time taken by microneedles to dissolve was measured after application into the murine skin. The ability of MN to form pores in the murine skin was evaluated by methylene blue staining. The in vivo efficacy of the vaccine MPs loaded-MNs was tested in the preclinical murine model after administering one prime and two booster doses. Control groups did not receive any treatment. Serum samples were collected periodically and were assessed for antibody titers using ELISA. Seven weeks after the last dose, animals were sacrificed and immune organs such as spleen and lymph nodes were collected. These organs were processed into single-cell suspensions and were evaluated for expression of helper (CD4) and cytotoxic T (CD8) cell markers.
Results: The size of vaccine MPs was found to be 573.4 ± 10.18 nm with a polydispersity index of 0.294 ± 0.133. The zeta potential of vaccine MP was found to be -22.6 ± 0.503 mV. The encapsulation efficiency was in the range of 55-70%. Scanning electron microscopic images showed that the MPs were spherical. The average length of microneedles was found to be 387 µm. In vitro release study showed that 50% of the encapsulated antigen was released in 24 hours and the next 20% was released over a week exhibiting sustained release. MNs dissolved within 10 minutes of application into the murine skin and were able to form pores as observed through methylene blue staining. The group of mice receiving vaccine MPs with or without adjuvants produced significantly higher antibody titers (total IgG as well as subtypes IgG1, IgG2a, and IgG3) when compared to the control group. IgG1 and IgG2a are essential in stimulating Th2 and Th1 mediated immune responses while IgG3 is critical in initiating effector immune mechanisms and neutralization. The group receiving blank MPs-loaded MNs did not produce significant antibody titer indicating that the MP and MN matrices were not immunogenic. The T cells isolated from immune organs of the treatment groups showed significantly higher expression of helper (CD4) and cytotoxic (CD8a) cell surface markers.
Conclusion: Zika vaccine MPs-loaded dissolving MN were formulated and were able to induce a robust antibody-mediated and cell-mediated immune response in a preclinical murine model. Induction of antibody titers as well as T cell surface markers demonstrated stimulation of both the arms of the adaptive immune system. Thus, this study provided a proof of concept for a pain-free vaccine against Zika.
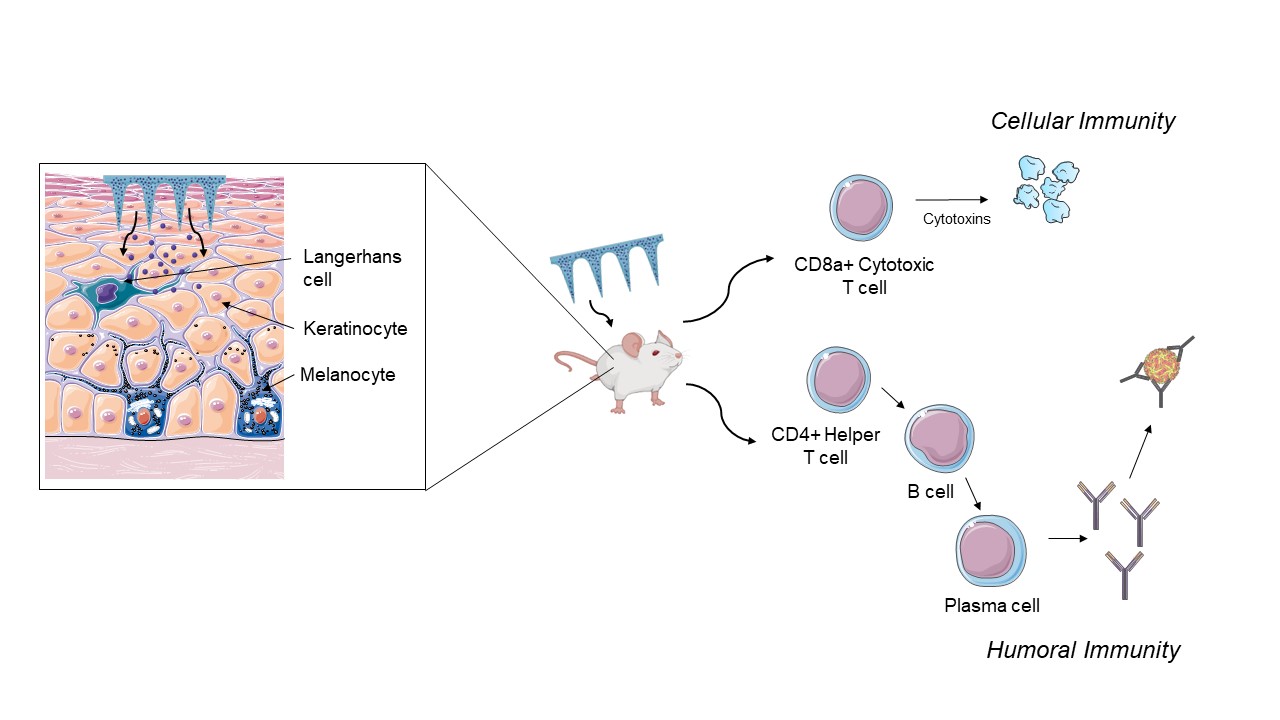
Figure 1: Schematic of Zika vaccine microparticles-loaded dissolving microneedles stimulating the antibody-mediated and cell-mediated immune response in a preclinical murine model. Langerhans cells are a type of immune cells present in the skin that help in the uptake of vaccine and initiate the cascade of immune response.
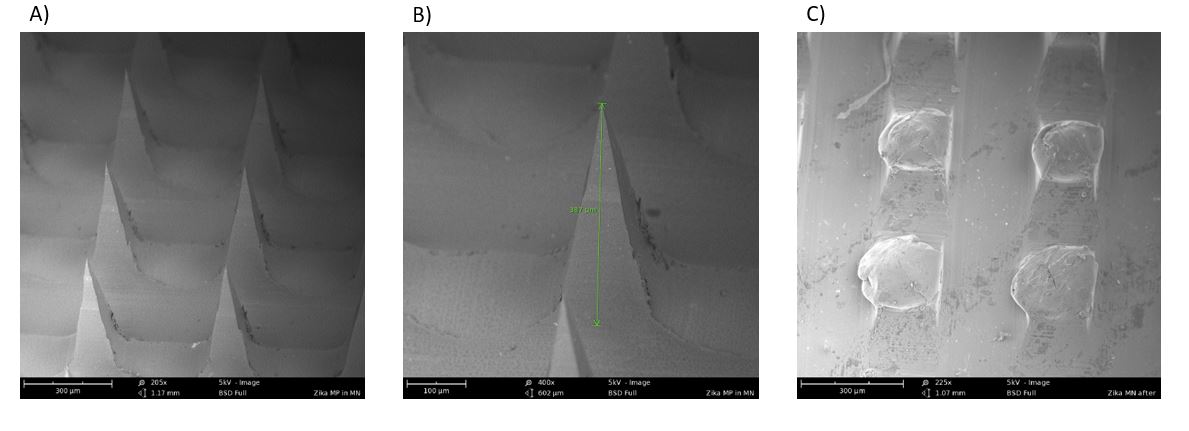
Figure 2: Scanning electron microscopic images of A) Microneedles in the array (205X); B) A single microneedle in the array with a length of 387 um (400X); C) Dissolved microneedles after application into the murine skin for 10 minutes (225X)
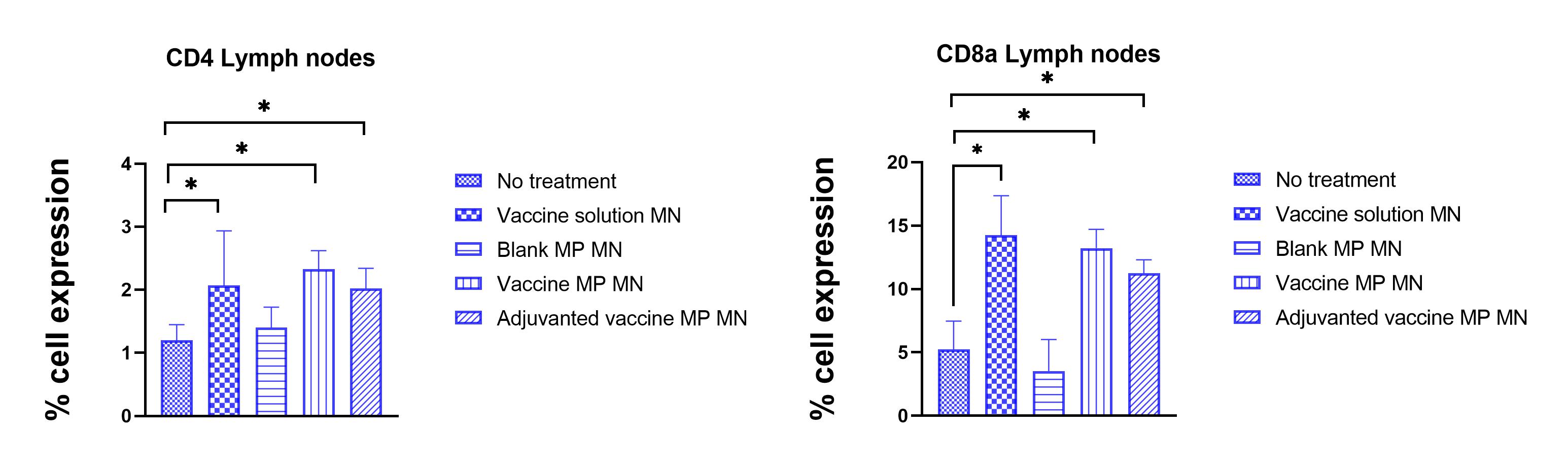
Figure 3: Percent expression of helper (CD4+) and cytotoxic (CD8a) cell surface markers in T cells isolated from lymph nodes of control and treatment groups. It was observed that T cells in lymph nodes of groups receiving vaccine solution, vaccine microparticles-loaded MNs showed significantly higher expression of CD4 and CD8a markers. (N<= 5, Normality of the data was tested by Shapiro-Wilk test then data was analyzed using One-Way ANOVA followed by Dunnett's multiple comparison test, * P < 0.05)
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(W1030-12-68) No Pain, Immunity Gain: Pain-Free Dissolving Microneedles as a Vaccine Strategy against Zika
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- AK
Akanksha Kale, BS
Mercer University
Atlanta, Georgia, United States - AK
Akanksha Kale, BS
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Zika is a zoonotic disease caused by the Zika virus and transmitted to humans through mosquito bites. Researchers have observed a causal relationship between Zika infection and congenital birth defects in children born to infected mothers. Zika infection can also result in Guillain-Barr Syndrome, an autoimmune disorder affecting the peripheral nervous system. Unfortunately, there is a lack of an approved vaccine against Zika to date. Current vaccine modalities under research are administered via either subcutaneous or intramuscular injection. These are painful and reduce patient compliance. Moreover, Zika is endemic in developing countries where the distribution of vaccines is challenging due to the scarcity of cold-chain storage resources and trained personnel. Formulating a vaccine in dry powder form would enhance the stability and eliminate the need for cold-chain storage. Therefore, in this study, we have formulated Zika vaccine microparticles (MPs)-loaded dissolving microneedles (MNs) to provide a pain-free immunization alternative to conventional vaccines. We evaluated the efficacy of this vaccine in a preclinical mouse model by measuring antibody titers and cellular immune response.
Methods: The inactivated Zika virus-loaded vaccine MPs were formulated by a double emulsion solvent evaporation method followed by lyophilization using poly(lactic-co-glycolic) acid (PLGA) as the polymer. Adjuvant MPs encapsulating a combination of Alhydrogel® and MPL-A® were formulated following a similar method. After testing for size, charge, morphology, encapsulation efficiency, and in vitro release, the vaccine MPs with or without adjuvant were loaded into dissolving MNs. The average needle length of MNs was measured using scanning electron microscopy (SEM). The time taken by microneedles to dissolve was measured after application into the murine skin. The ability of MN to form pores in the murine skin was evaluated by methylene blue staining. The in vivo efficacy of the vaccine MPs loaded-MNs was tested in the preclinical murine model after administering one prime and two booster doses. Control groups did not receive any treatment. Serum samples were collected periodically and were assessed for antibody titers using ELISA. Seven weeks after the last dose, animals were sacrificed and immune organs such as spleen and lymph nodes were collected. These organs were processed into single-cell suspensions and were evaluated for expression of helper (CD4) and cytotoxic T (CD8) cell markers.
Results: The size of vaccine MPs was found to be 573.4 ± 10.18 nm with a polydispersity index of 0.294 ± 0.133. The zeta potential of vaccine MP was found to be -22.6 ± 0.503 mV. The encapsulation efficiency was in the range of 55-70%. Scanning electron microscopic images showed that the MPs were spherical. The average length of microneedles was found to be 387 µm. In vitro release study showed that 50% of the encapsulated antigen was released in 24 hours and the next 20% was released over a week exhibiting sustained release. MNs dissolved within 10 minutes of application into the murine skin and were able to form pores as observed through methylene blue staining. The group of mice receiving vaccine MPs with or without adjuvants produced significantly higher antibody titers (total IgG as well as subtypes IgG1, IgG2a, and IgG3) when compared to the control group. IgG1 and IgG2a are essential in stimulating Th2 and Th1 mediated immune responses while IgG3 is critical in initiating effector immune mechanisms and neutralization. The group receiving blank MPs-loaded MNs did not produce significant antibody titer indicating that the MP and MN matrices were not immunogenic. The T cells isolated from immune organs of the treatment groups showed significantly higher expression of helper (CD4) and cytotoxic (CD8a) cell surface markers.
Conclusion: Zika vaccine MPs-loaded dissolving MN were formulated and were able to induce a robust antibody-mediated and cell-mediated immune response in a preclinical murine model. Induction of antibody titers as well as T cell surface markers demonstrated stimulation of both the arms of the adaptive immune system. Thus, this study provided a proof of concept for a pain-free vaccine against Zika.

Figure 1: Schematic of Zika vaccine microparticles-loaded dissolving microneedles stimulating the antibody-mediated and cell-mediated immune response in a preclinical murine model. Langerhans cells are a type of immune cells present in the skin that help in the uptake of vaccine and initiate the cascade of immune response.

Figure 2: Scanning electron microscopic images of A) Microneedles in the array (205X); B) A single microneedle in the array with a length of 387 um (400X); C) Dissolved microneedles after application into the murine skin for 10 minutes (225X)

Figure 3: Percent expression of helper (CD4+) and cytotoxic (CD8a) cell surface markers in T cells isolated from lymph nodes of control and treatment groups. It was observed that T cells in lymph nodes of groups receiving vaccine solution, vaccine microparticles-loaded MNs showed significantly higher expression of CD4 and CD8a markers. (N<= 5, Normality of the data was tested by Shapiro-Wilk test then data was analyzed using One-Way ANOVA followed by Dunnett's multiple comparison test, * P < 0.05)
