Back
Purpose: Interindividual differences in response to drugs, like age, pharmacogenetic profiles and pharmacokinetic characteristics raise a demand for personalized therapeutic formulations [1-3]. 3D printing holds great promise in delivering personalized and on-demand treatment regimen [4]. Newly emerged pharmaceutical agents are mostly biomacromolecules such as proteins and polynucleotides. Despite excellent therapeutic effectiveness, these agents are associated with serious challenges and limitations. Encapsulation of the API in polymeric particles is a major strategy to deal with encountered challenges. To meet the high demand for these particles, a robust technology is required for massive fabrication of high quality and controllable particles. Herein, we aim to develop a platform to make drug loaded microparticles (µP) using advanced 3D printing techniques. This novel method, sprayed multi adsorbed-droplet reposing technology (SMART), employs pneumatic pressure assisted, extrusion-based 3D printing and emulsion evaporation for fabricating µP-based drug delivery systems encapsulating an active pharmaceutical ingredient.
Methods: In this work, we incorporated the model protein, ovalbumin (OVA), within the PLGA µPs as a drug delivery system for pharmaceutical agents through SMART process. In this regard, OVA was dissolved in PBS buffer containing poly(vinyl alcohol) (PVA) solution (0.5 % w/w) as the inner aqueous phase. PLGA was dissolved in dichloromethane (DCM) to form the organic phase. Inner aqueous phase was added into the organic phase (W/O volume ration 1:4) and the mixture was then extruded through Cellink BioX 3D printer (Pneumatic print-head, 200 kPa pressure, 25-gauge nozzle) to obtain a homogenous primary emulsion. The primary emulsion was then added into 0.5% PVA solution (W/O/W volume ratio of 1:4:16), followed by extrusion through the bioprinter (Pneumatic print-head, various pressures, 25-gauge nozzle) to generate a secondary emulsion. After the evaporation of the organic solvent for one hour in a desiccator under vacuum, the OVA-loaded µPs were separated and washed by ultracentrifugation. µPs were then collected and characterized using various characterization techniques.
Results: The particles varied in size as the pressure and extrusion cycles changed. The process exhibited a high yield of drug-loaded µPs, 78.3 ± 9.4%. The encapsulation efficiency and loading were determined to be 84.8 ± 4.3% (w/w) and 13.4 ± 0.7% for the best formulation, respectively. SEM images confirmed the spherical morphology of the particles and the effect of pressure and extrusion cycles on µPs morphology. FTIR and CD spectrums revealed intact chemical structure, and secondary conformational structure of the OVA molecule after processing and encapsulation, respectively. Figure 1 summarizes the data.
Conclusion: SAMRT technique provided for encapsulation of a variety of substances including the model protein (OVA) into polymeric µP with controllable properties and is suitable for on demand manufacturing of personalized formulations taking advantage of additive manufacturing technologies.
References: [1] Guzzi, Elia A., and Mark W. Tibbitt. "Additive manufacturing of precision biomaterials." Advanced Materials 32.13 (2020): 1901994.
[2] Gioumouxouzis, Christos I., Christina Karavasili, and Dimitrios G. Fatouros. "Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies." Drug Discovery Today 24.2 (2019): 636-643.
[3] Borandeh, Sedigheh, et al. "Polymeric drug delivery systems by additive manufacturing." Advanced Drug Delivery Reviews 173 (2021): 349-373.
[4] Wang, Jiawei, et al. "Emerging 3D printing technologies for drug delivery devices: Current status and future perspective." Advanced Drug Delivery Reviews 174 (2021): 294-316.
Acknowledgements: This work is funded by the National Institute of Health (NIH), Grant number #R01FD007456.
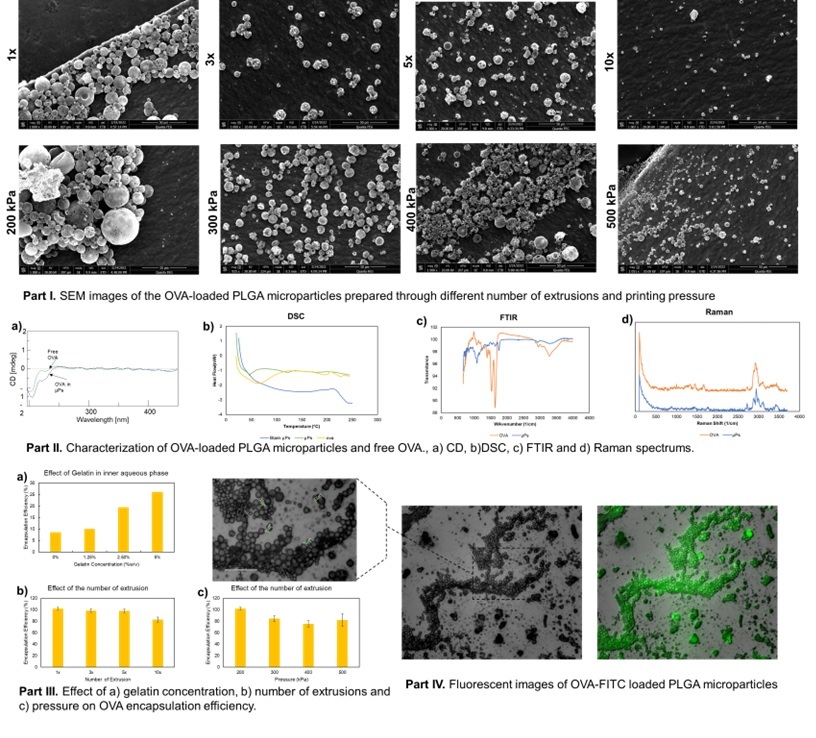
Figure 1: Product Characterization and Optimization
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(W0930-07-38) Sprayed Multi-Adsorbed Droplet Reposing Technology (SMART) for Fabrication of a Protein Drug Delivery System
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- NH
Niloofar Heshmati, Ph.D.
University of Texas at Austin
Austin, Texas, United States - NH
Niloofar Heshmati, Ph.D.
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: Interindividual differences in response to drugs, like age, pharmacogenetic profiles and pharmacokinetic characteristics raise a demand for personalized therapeutic formulations [1-3]. 3D printing holds great promise in delivering personalized and on-demand treatment regimen [4]. Newly emerged pharmaceutical agents are mostly biomacromolecules such as proteins and polynucleotides. Despite excellent therapeutic effectiveness, these agents are associated with serious challenges and limitations. Encapsulation of the API in polymeric particles is a major strategy to deal with encountered challenges. To meet the high demand for these particles, a robust technology is required for massive fabrication of high quality and controllable particles. Herein, we aim to develop a platform to make drug loaded microparticles (µP) using advanced 3D printing techniques. This novel method, sprayed multi adsorbed-droplet reposing technology (SMART), employs pneumatic pressure assisted, extrusion-based 3D printing and emulsion evaporation for fabricating µP-based drug delivery systems encapsulating an active pharmaceutical ingredient.
Methods: In this work, we incorporated the model protein, ovalbumin (OVA), within the PLGA µPs as a drug delivery system for pharmaceutical agents through SMART process. In this regard, OVA was dissolved in PBS buffer containing poly(vinyl alcohol) (PVA) solution (0.5 % w/w) as the inner aqueous phase. PLGA was dissolved in dichloromethane (DCM) to form the organic phase. Inner aqueous phase was added into the organic phase (W/O volume ration 1:4) and the mixture was then extruded through Cellink BioX 3D printer (Pneumatic print-head, 200 kPa pressure, 25-gauge nozzle) to obtain a homogenous primary emulsion. The primary emulsion was then added into 0.5% PVA solution (W/O/W volume ratio of 1:4:16), followed by extrusion through the bioprinter (Pneumatic print-head, various pressures, 25-gauge nozzle) to generate a secondary emulsion. After the evaporation of the organic solvent for one hour in a desiccator under vacuum, the OVA-loaded µPs were separated and washed by ultracentrifugation. µPs were then collected and characterized using various characterization techniques.
Results: The particles varied in size as the pressure and extrusion cycles changed. The process exhibited a high yield of drug-loaded µPs, 78.3 ± 9.4%. The encapsulation efficiency and loading were determined to be 84.8 ± 4.3% (w/w) and 13.4 ± 0.7% for the best formulation, respectively. SEM images confirmed the spherical morphology of the particles and the effect of pressure and extrusion cycles on µPs morphology. FTIR and CD spectrums revealed intact chemical structure, and secondary conformational structure of the OVA molecule after processing and encapsulation, respectively. Figure 1 summarizes the data.
Conclusion: SAMRT technique provided for encapsulation of a variety of substances including the model protein (OVA) into polymeric µP with controllable properties and is suitable for on demand manufacturing of personalized formulations taking advantage of additive manufacturing technologies.
References: [1] Guzzi, Elia A., and Mark W. Tibbitt. "Additive manufacturing of precision biomaterials." Advanced Materials 32.13 (2020): 1901994.
[2] Gioumouxouzis, Christos I., Christina Karavasili, and Dimitrios G. Fatouros. "Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies." Drug Discovery Today 24.2 (2019): 636-643.
[3] Borandeh, Sedigheh, et al. "Polymeric drug delivery systems by additive manufacturing." Advanced Drug Delivery Reviews 173 (2021): 349-373.
[4] Wang, Jiawei, et al. "Emerging 3D printing technologies for drug delivery devices: Current status and future perspective." Advanced Drug Delivery Reviews 174 (2021): 294-316.
Acknowledgements: This work is funded by the National Institute of Health (NIH), Grant number #R01FD007456.

Figure 1: Product Characterization and Optimization
