Back
Purpose: Psychosis is a prominent symptom in schizophrenia, bipolar disorders, and neuropsychiatric manifestation of Alzheimer's disease, occurring in approximately 50% of patients affected by the same1. There is an unmet medical need to develop a less user-dependent long-acting product for the treatment of psychosis. Olanzapine (OZP) is a safe and effective atypical antipsychotic drug used in treating psychosis associated with schizophrenia and bipolar disorders. The dosage forms currently on the market for OZP are administered through oral or intravenous (IV) routes. However, despite the benefits of OZP, there are many problems associated with oral and IV routes of drug administration, such as first-pass metabolism, peak and valley plasma drug concentrations, and invasiveness of IV route, leading to poor patient adherence. Thus, we aimed to develop a drug-in-adhesive transdermal delivery system (TDS) that can deliver OZP for three days. The TDS will be non-invasive, improve patient adherence, and have high clinical relevance.
Methods: We determined passive permeation and the effect of oleic acid as a chemical enhancer on the OZP delivery across skin. We also studied the delivery of OZP across different skin types such as dermatomed porcine skin, dermatomed human skin, and heat-separated human epidermis. Crystallization studies were conducted to determine the saturation solubility of OZP in three pressure-sensitive adhesives (PSA) to optimize delivery and avoid the risk of the drug crystallizing out in the adhesive. We optimized various parameters such as drying time, selection of backing membrane, and release liner to formulate a solution-based TDS in acrylate PSA and a suspension-based TDS in silicone and polyisobutylene (PIB) PSA with oleic acid as a chemical enhancer. In vitro permeation testing was conducted to determine the amount of OZP permeated from TDSs using heat-separated human epidermis on vertical Franz diffusion cells. The amount of OZP in the receptor samples was analyzed using a validated HPLC method. In vitro EpiDermTM skin irritation test (EPI-200-SIT) study based on a three-dimensional reconstructed human epidermis model (RhE) for in vitro tissue culture and a cell viability test, was conducted for PIB TDA to check any skin irritation potential.
Results: Amount of OZP delivered across the skin from a solution of 10% w/w oleic acid in propylene glycol as a chemical enhancer (1181.71 ± 179.18 µg/cm2) exceeded the desired target delivery for three days (180 µg/cm2) and was significantly higher than passive permeation (17.68 ± 2.16 µg/cm2). There was no significant difference in the amount of OZP delivered across dermatomed porcine skin, dermatomed human skin, and heat-separated human epidermis. The solubility of OZP in acrylate PSA was 3.8% w/w but less than 0.1% w/w in silicone and polyisobutylene (PIB) PSA (Figure 2). Hence a solution-based TDS was developed in acrylate PSA, while a suspension-based TDS was developed in silicone and PIB PSA. 3M™ CoTran™ Ethylene Vinyl Acetate Membrane Film 9702 was selected as the backing membrane, while 3M Scotchpak™ 9744 Release Liner Fluoropolymer Coated Polyester Film was chosen as the release liner for solution as well as suspension based TDS. Air drying time of 30 minutes was optimized. The solution and suspension-based PSA TDS were characterized for coat weight, shear strength, tack, and peel adhesion. In vitro permeation testing studies indicated that acrylate solution-based TDS, silicone and PIB suspension-based TDS delivered 58.97 ± 6.59 µg/sq.cm, 129.34 ± 16.59 µg/sq.cm, and 245.00 ± 2.51 µg/sq.cm of OZP respectively (Figure 3). Thus, PIB PSA suspension-based TDS met the desired target delivery for three days. Skin irritation testing studies using the In vitro EpiDermTM skin irritation test reported that PIB TDS was non-irritant.
Conclusion: Acrylate solution-based TDS, silicone, and PIB suspension-based TDS successfully delivered OZP. PIB suspension-based TDS met the desired target delivery for three days and was also non-irritant to skin. Thus, the PIB PSA suspension-based PSA could serve as a potentially effective transdermal delivery system for olanzapine.
References: 1. Murray, P. S., Kumar, S., Demichele-Sweet, M. A., & Sweet, R. A. (2014). Psychosis in Alzheimer's disease. Biological psychiatry, 75(7), 542–552. https://doi.org/10.1016/j.biopsych.2013.08.020
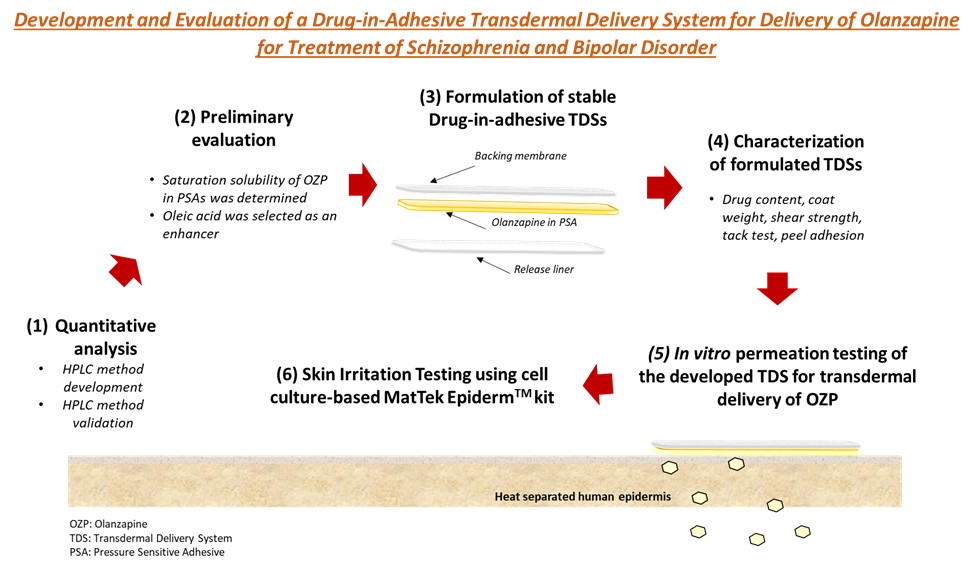
Figure 1: Schematic representing studies conducted to develop and evaluate the transdermal delivery system of olanzapine.
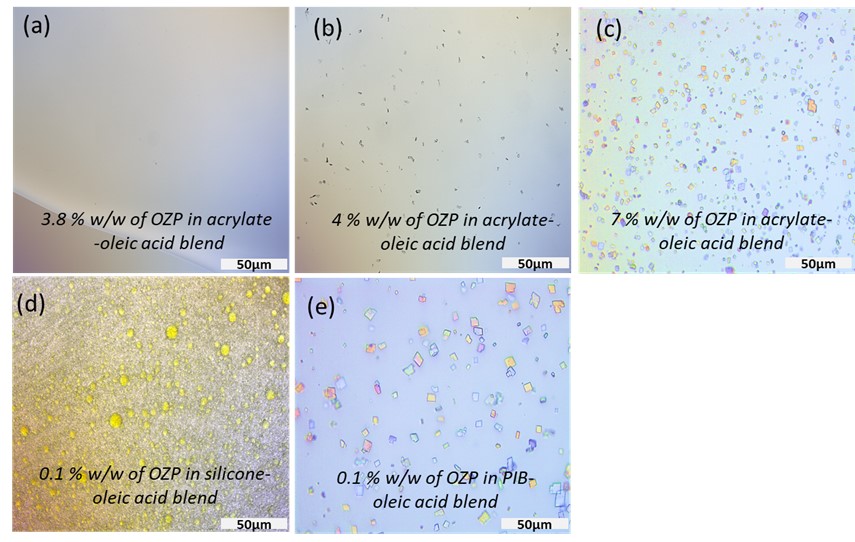
Figure 2: Images of slide crystallization studies using a polarized light microscope (a) No crystals were observed in 3.8 % w/w of OZP in acrylate-oleic acid blend (b) crystals of OZP in 4 % w/w of OZP in acrylate-oleic acid blend (c) crystals of OZP in 7 % w/w of OZP in acrylate-oleic acid blend (d) 0.1 % w/w of OZP in silicone-oleic acid blend (e) crystals of OZP in 0.1 % w/w of OZP in PIB-oleic acid blend. The scale bar represents 50 µm.
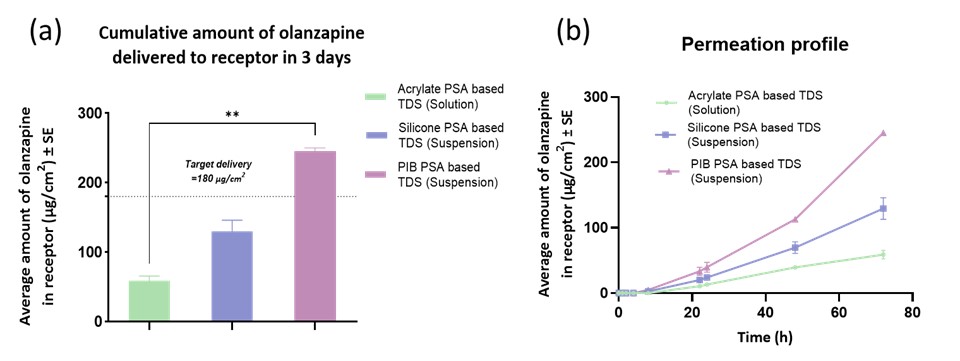
Figure 3: Results from in vitro permeation testing from solution and suspension-based TDS (a) Cumulative amount of olanzapine delivered to receptor in 3 days (b) Permeation profile of amount of olanzapine delivered in 3 days. Values are expressed as an average amount of olanzapine (µg/cm2) ± SE and n=4; Statistical analysis performed by Kruskal-Wallis test, ** indicates statistical difference between groups, p < 0.01.
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(W0930-04-23) Development and Evaluation of a Drug-in-Adhesive Transdermal Delivery System for Delivery of Olanzapine for Treatment of Schizophrenia and Bipolar Disorder
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET

Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Psychosis is a prominent symptom in schizophrenia, bipolar disorders, and neuropsychiatric manifestation of Alzheimer's disease, occurring in approximately 50% of patients affected by the same1. There is an unmet medical need to develop a less user-dependent long-acting product for the treatment of psychosis. Olanzapine (OZP) is a safe and effective atypical antipsychotic drug used in treating psychosis associated with schizophrenia and bipolar disorders. The dosage forms currently on the market for OZP are administered through oral or intravenous (IV) routes. However, despite the benefits of OZP, there are many problems associated with oral and IV routes of drug administration, such as first-pass metabolism, peak and valley plasma drug concentrations, and invasiveness of IV route, leading to poor patient adherence. Thus, we aimed to develop a drug-in-adhesive transdermal delivery system (TDS) that can deliver OZP for three days. The TDS will be non-invasive, improve patient adherence, and have high clinical relevance.
Methods: We determined passive permeation and the effect of oleic acid as a chemical enhancer on the OZP delivery across skin. We also studied the delivery of OZP across different skin types such as dermatomed porcine skin, dermatomed human skin, and heat-separated human epidermis. Crystallization studies were conducted to determine the saturation solubility of OZP in three pressure-sensitive adhesives (PSA) to optimize delivery and avoid the risk of the drug crystallizing out in the adhesive. We optimized various parameters such as drying time, selection of backing membrane, and release liner to formulate a solution-based TDS in acrylate PSA and a suspension-based TDS in silicone and polyisobutylene (PIB) PSA with oleic acid as a chemical enhancer. In vitro permeation testing was conducted to determine the amount of OZP permeated from TDSs using heat-separated human epidermis on vertical Franz diffusion cells. The amount of OZP in the receptor samples was analyzed using a validated HPLC method. In vitro EpiDermTM skin irritation test (EPI-200-SIT) study based on a three-dimensional reconstructed human epidermis model (RhE) for in vitro tissue culture and a cell viability test, was conducted for PIB TDA to check any skin irritation potential.
Results: Amount of OZP delivered across the skin from a solution of 10% w/w oleic acid in propylene glycol as a chemical enhancer (1181.71 ± 179.18 µg/cm2) exceeded the desired target delivery for three days (180 µg/cm2) and was significantly higher than passive permeation (17.68 ± 2.16 µg/cm2). There was no significant difference in the amount of OZP delivered across dermatomed porcine skin, dermatomed human skin, and heat-separated human epidermis. The solubility of OZP in acrylate PSA was 3.8% w/w but less than 0.1% w/w in silicone and polyisobutylene (PIB) PSA (Figure 2). Hence a solution-based TDS was developed in acrylate PSA, while a suspension-based TDS was developed in silicone and PIB PSA. 3M™ CoTran™ Ethylene Vinyl Acetate Membrane Film 9702 was selected as the backing membrane, while 3M Scotchpak™ 9744 Release Liner Fluoropolymer Coated Polyester Film was chosen as the release liner for solution as well as suspension based TDS. Air drying time of 30 minutes was optimized. The solution and suspension-based PSA TDS were characterized for coat weight, shear strength, tack, and peel adhesion. In vitro permeation testing studies indicated that acrylate solution-based TDS, silicone and PIB suspension-based TDS delivered 58.97 ± 6.59 µg/sq.cm, 129.34 ± 16.59 µg/sq.cm, and 245.00 ± 2.51 µg/sq.cm of OZP respectively (Figure 3). Thus, PIB PSA suspension-based TDS met the desired target delivery for three days. Skin irritation testing studies using the In vitro EpiDermTM skin irritation test reported that PIB TDS was non-irritant.
Conclusion: Acrylate solution-based TDS, silicone, and PIB suspension-based TDS successfully delivered OZP. PIB suspension-based TDS met the desired target delivery for three days and was also non-irritant to skin. Thus, the PIB PSA suspension-based PSA could serve as a potentially effective transdermal delivery system for olanzapine.
References: 1. Murray, P. S., Kumar, S., Demichele-Sweet, M. A., & Sweet, R. A. (2014). Psychosis in Alzheimer's disease. Biological psychiatry, 75(7), 542–552. https://doi.org/10.1016/j.biopsych.2013.08.020

Figure 1: Schematic representing studies conducted to develop and evaluate the transdermal delivery system of olanzapine.

Figure 2: Images of slide crystallization studies using a polarized light microscope (a) No crystals were observed in 3.8 % w/w of OZP in acrylate-oleic acid blend (b) crystals of OZP in 4 % w/w of OZP in acrylate-oleic acid blend (c) crystals of OZP in 7 % w/w of OZP in acrylate-oleic acid blend (d) 0.1 % w/w of OZP in silicone-oleic acid blend (e) crystals of OZP in 0.1 % w/w of OZP in PIB-oleic acid blend. The scale bar represents 50 µm.

Figure 3: Results from in vitro permeation testing from solution and suspension-based TDS (a) Cumulative amount of olanzapine delivered to receptor in 3 days (b) Permeation profile of amount of olanzapine delivered in 3 days. Values are expressed as an average amount of olanzapine (µg/cm2) ± SE and n=4; Statistical analysis performed by Kruskal-Wallis test, ** indicates statistical difference between groups, p < 0.01.
