Back
Purpose: Selective phosphodiesterase (PDE) 4 inhibitors have been developed as potent anti-inflammatory drugs. Despite the licensing of roflumilast (RFM) tablets to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, its use has been severely hindered by the dose-limiting off-target side effects. The potential of nanoparticles to sustain drug delivery to the lungs after inhalation is well recognised. The targeted delivery provided can potentially result in prolonged drug exposure at the target site to achieve an optimised therapeutic effect. Moreover, delivery of drug directly to the site of action reduces systemic exposure and therefore off-target drug action. This work aimed to establish methods of a disease model and aerosol delivery for free and encapsulated roflumilast for exploring the potential of inhalation as a delivery strategy for PDE4 inhibitors to improve their therapeutic window.
Methods: Lipopolysaccharide-induced acute lung injury model using a whole-body chamber: Male Dunkin-Hartley guinea pigs (250 - 299 g) were placed in a whole-body chamber and were sensitized with increasing concentration of lipopolysaccharide (LPS) (0.1, 0.2 and 0.5 mg/mL) via nebulization using an Aerogen® Pro nebuliser (Aerogen Ireland Ltd., S/N 356, volume median diameter with saline 0.9% 4.82 µm, average flow rate 0.38 mL/min). Four hours later, the animals were euthanized and the cell populations in the bronchoalveolar lavage fluid (BALF) were identified on air-dried cytocentrifuged smears after staining with Diff-Quick stain. Differential cell counts were performed on 200 total cells using four different fields of view. Optimization of RFM exposure in LPS-challenged guinea pigs using a nebulisation approach: Roflumilast was suspended in saline/0.2% Tween 80 + 0.05% DMSO and particle shape and geometric size distribution evaluated using Morphologi 4. Roflumilast (100 µg/mL, 4 mL and 500 µg/mL, 2 mL) suspensions were nebulized using an Aerogen® Pro nebuliser and the aerodynamic particle size of the aerosol from the nebuliser determined using the Next Generation Impactor (NGI, flow rate 15 L/min). Roflumilast mass fractional stage deposition from the NGI was analysed using an high-performance liquid chromatography (HPLC) method. Male Dunkin-Hartley guinea pigs (250 - 299 g) were exposed to nebulised roflumilast in saline/0.2% Tween 80 + 0.05% DMSO (delivered dose, DD: 50 µg/kg) at t = 0 min followed by exposure to nebulised lipopolysaccharide (0.2 mg/mL) at t = 60 min. Four hours after the lipopolysaccharide challenge, animals were euthanized and bronchoalveolar lavage (BAL) performed. Total and differential cell counts were carried out on BALF. Effect of nebulisation on physiochemical properties of albumin NPs: A 15 mL falcon tube was connected to the inlet of a 15 mm ISO t-piece. A fixed volume of nanoparticles (NPs, 0.4 mL) was nebulised for 1 min and the condensed fluid in the falcon tube was recovered via the addition of 1 mL of deionized water. Physiochemical properties were evaluated with Dynamic Light Scattering.
Results: The in vivo effects of the LPS stimulation via nebulisation using a whole-body chamber model were investigated. The exposure to increasing concentrations of LPS resulted in a dose-dependent increase of the total cell count (TCC) (175.0 ± 112.4, 324.3 ± 52.0, 350.3 ± 2.5 x 104 cells/mL, respectively, for 0.1, 0.2 and 0.5 mg/mL) and neutrophil number recovered in the BALF (128.8 ± 84.7, 291.7 ± 24.3, 294.7 ± 2.8 x 104 cells/mL respectively for 0.1, 0.2 and 0.5 mg/mL) (Figure 1). The drug particles in the suspension were irregularly shaped. The nebulisation of the drug suspension resulted in aerodynamic characteristics suitable for further in vivo testing. The fine particle fraction (FPF) was 35% and 31% for the 100 and 500 µg/mL, respectively, with the highest proportion of roflumilast recovered in the stages 2, 3 and 4 (cut-off diameter: 8.61, 5.39 and 3.30 µm respectively) of the impactor. In preliminary experiments nebulised drug did not produce significant reduction of the neutrophil influx in the BALF, indicating that either an increase in the delivered dose or longer exposure to drug may be required for anti-inflammatory activity. As a first step towards investigating the use of inhaled albumin nanoparticles as a delivery strategy, the impact of nebulisation on the physiochemical properties of the nanoparticles was investigated. Promisingly, the nebulisation of NPs did not change particle size, PDI or zeta potential (Table 1).
Conclusion: This study established an in vivo nebulisation of lipopolysaccharide (0.2 mg/mL) to guinea pigs using a whole-body chamber model as a robust acute lung inflammation model. A respirable roflumilast suspension was developed but did not significantly reduce the lung inflammation after nebulisation of 50 µg/kg. However, albumin nanoparticles have been shown to remain physiochemically unchanged after nebulisation and will be utilised in future studies to evaluate their utility as an inhaled drug delivery system for roflumilast.
Acknowledgements: All experiments were performed at King's College London according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines, the UK Animals (Scientific Procedures) Act 1986, and the 2012 amendments, and were approved by the King's College London ethics committee. The animals were housed in rooms under controlled temperature (22 ± 1 °C), humidity (55 ± 10%), and 12-h light/dark cycle. Food and water were available at all times.
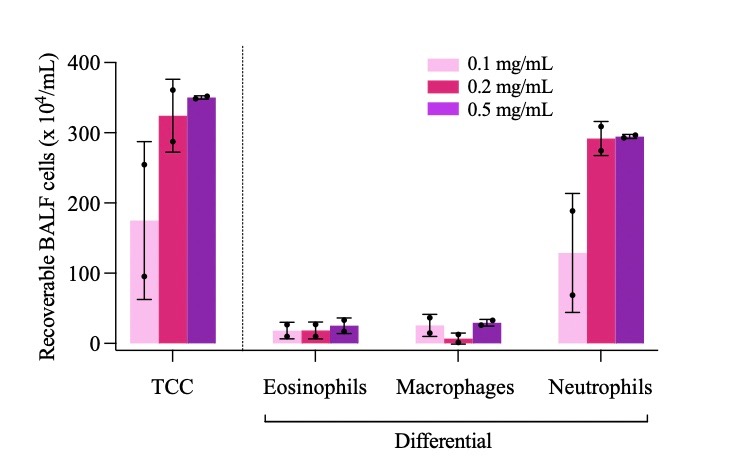
Figure 1. Total (TCC) and differential cell count from bronchoalveolar lavage fluid (BALF) of guinea pigs exposed to increasing concentration of lipopolysaccharide from E. Coli. Cells were recovered four hours after the inflammatory challenge. Cell populations were identified on air-dried cytocentrifuged smears after staining with Diff-Quick stain. Differential cell counts were performed on 200 total cells using four different fields of view. Data represent mean ± SD of n = 2 animals per group.
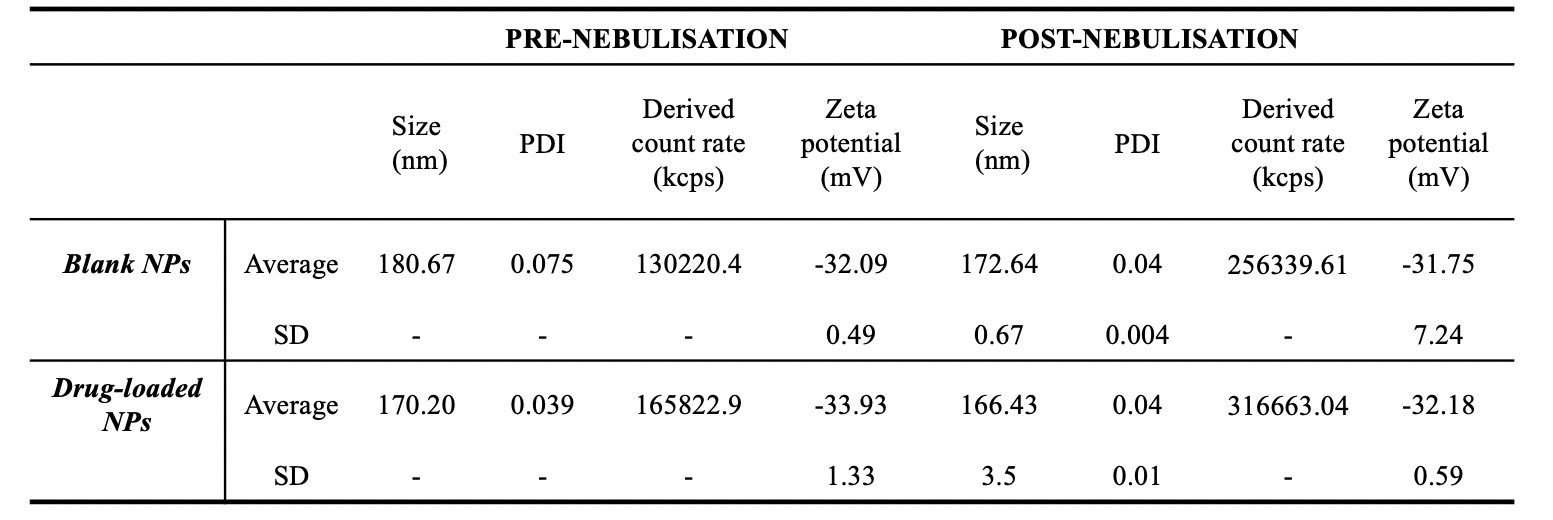
Table 1. Size, polydispersity index (PDI) and zeta potential of blank and drug loaded nanoparticles (NPs) prior and after nebulisation with Aerogen Pro®. A 15 mL falcon tube was connected with tape to the inlet of a 15 mm ISO t-piece. A fixed volume of nanoparticles (0.4 mL) was nebulised for 1 min and the condensation fluid in the falcon tube was recovered via the addition of 1 mL of deionized water. Physiochemical properties were evaluated with Dynamic Light Scattering. Data represent values from one batch of nanoparticles nebulised n=1 – 4.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1430-12-70) Inhaled Albumin Nanoparticles as a Delivery Strategy to Improve the Risk/Benefit Ratio of PDE-4 Inhibitors
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET
- IS
Ivana Stolfa
King's College London
LONDON, England, United Kingdom - IS
Ivana Stolfa
King's College London
LONDON, England, United Kingdom
Presenting Author(s)
Main Author(s)
Purpose: Selective phosphodiesterase (PDE) 4 inhibitors have been developed as potent anti-inflammatory drugs. Despite the licensing of roflumilast (RFM) tablets to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, its use has been severely hindered by the dose-limiting off-target side effects. The potential of nanoparticles to sustain drug delivery to the lungs after inhalation is well recognised. The targeted delivery provided can potentially result in prolonged drug exposure at the target site to achieve an optimised therapeutic effect. Moreover, delivery of drug directly to the site of action reduces systemic exposure and therefore off-target drug action. This work aimed to establish methods of a disease model and aerosol delivery for free and encapsulated roflumilast for exploring the potential of inhalation as a delivery strategy for PDE4 inhibitors to improve their therapeutic window.
Methods: Lipopolysaccharide-induced acute lung injury model using a whole-body chamber: Male Dunkin-Hartley guinea pigs (250 - 299 g) were placed in a whole-body chamber and were sensitized with increasing concentration of lipopolysaccharide (LPS) (0.1, 0.2 and 0.5 mg/mL) via nebulization using an Aerogen® Pro nebuliser (Aerogen Ireland Ltd., S/N 356, volume median diameter with saline 0.9% 4.82 µm, average flow rate 0.38 mL/min). Four hours later, the animals were euthanized and the cell populations in the bronchoalveolar lavage fluid (BALF) were identified on air-dried cytocentrifuged smears after staining with Diff-Quick stain. Differential cell counts were performed on 200 total cells using four different fields of view. Optimization of RFM exposure in LPS-challenged guinea pigs using a nebulisation approach: Roflumilast was suspended in saline/0.2% Tween 80 + 0.05% DMSO and particle shape and geometric size distribution evaluated using Morphologi 4. Roflumilast (100 µg/mL, 4 mL and 500 µg/mL, 2 mL) suspensions were nebulized using an Aerogen® Pro nebuliser and the aerodynamic particle size of the aerosol from the nebuliser determined using the Next Generation Impactor (NGI, flow rate 15 L/min). Roflumilast mass fractional stage deposition from the NGI was analysed using an high-performance liquid chromatography (HPLC) method. Male Dunkin-Hartley guinea pigs (250 - 299 g) were exposed to nebulised roflumilast in saline/0.2% Tween 80 + 0.05% DMSO (delivered dose, DD: 50 µg/kg) at t = 0 min followed by exposure to nebulised lipopolysaccharide (0.2 mg/mL) at t = 60 min. Four hours after the lipopolysaccharide challenge, animals were euthanized and bronchoalveolar lavage (BAL) performed. Total and differential cell counts were carried out on BALF. Effect of nebulisation on physiochemical properties of albumin NPs: A 15 mL falcon tube was connected to the inlet of a 15 mm ISO t-piece. A fixed volume of nanoparticles (NPs, 0.4 mL) was nebulised for 1 min and the condensed fluid in the falcon tube was recovered via the addition of 1 mL of deionized water. Physiochemical properties were evaluated with Dynamic Light Scattering.
Results: The in vivo effects of the LPS stimulation via nebulisation using a whole-body chamber model were investigated. The exposure to increasing concentrations of LPS resulted in a dose-dependent increase of the total cell count (TCC) (175.0 ± 112.4, 324.3 ± 52.0, 350.3 ± 2.5 x 104 cells/mL, respectively, for 0.1, 0.2 and 0.5 mg/mL) and neutrophil number recovered in the BALF (128.8 ± 84.7, 291.7 ± 24.3, 294.7 ± 2.8 x 104 cells/mL respectively for 0.1, 0.2 and 0.5 mg/mL) (Figure 1). The drug particles in the suspension were irregularly shaped. The nebulisation of the drug suspension resulted in aerodynamic characteristics suitable for further in vivo testing. The fine particle fraction (FPF) was 35% and 31% for the 100 and 500 µg/mL, respectively, with the highest proportion of roflumilast recovered in the stages 2, 3 and 4 (cut-off diameter: 8.61, 5.39 and 3.30 µm respectively) of the impactor. In preliminary experiments nebulised drug did not produce significant reduction of the neutrophil influx in the BALF, indicating that either an increase in the delivered dose or longer exposure to drug may be required for anti-inflammatory activity. As a first step towards investigating the use of inhaled albumin nanoparticles as a delivery strategy, the impact of nebulisation on the physiochemical properties of the nanoparticles was investigated. Promisingly, the nebulisation of NPs did not change particle size, PDI or zeta potential (Table 1).
Conclusion: This study established an in vivo nebulisation of lipopolysaccharide (0.2 mg/mL) to guinea pigs using a whole-body chamber model as a robust acute lung inflammation model. A respirable roflumilast suspension was developed but did not significantly reduce the lung inflammation after nebulisation of 50 µg/kg. However, albumin nanoparticles have been shown to remain physiochemically unchanged after nebulisation and will be utilised in future studies to evaluate their utility as an inhaled drug delivery system for roflumilast.
Acknowledgements: All experiments were performed at King's College London according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines, the UK Animals (Scientific Procedures) Act 1986, and the 2012 amendments, and were approved by the King's College London ethics committee. The animals were housed in rooms under controlled temperature (22 ± 1 °C), humidity (55 ± 10%), and 12-h light/dark cycle. Food and water were available at all times.

Figure 1. Total (TCC) and differential cell count from bronchoalveolar lavage fluid (BALF) of guinea pigs exposed to increasing concentration of lipopolysaccharide from E. Coli. Cells were recovered four hours after the inflammatory challenge. Cell populations were identified on air-dried cytocentrifuged smears after staining with Diff-Quick stain. Differential cell counts were performed on 200 total cells using four different fields of view. Data represent mean ± SD of n = 2 animals per group.

Table 1. Size, polydispersity index (PDI) and zeta potential of blank and drug loaded nanoparticles (NPs) prior and after nebulisation with Aerogen Pro®. A 15 mL falcon tube was connected with tape to the inlet of a 15 mm ISO t-piece. A fixed volume of nanoparticles (0.4 mL) was nebulised for 1 min and the condensation fluid in the falcon tube was recovered via the addition of 1 mL of deionized water. Physiochemical properties were evaluated with Dynamic Light Scattering. Data represent values from one batch of nanoparticles nebulised n=1 – 4.
