Back
Purpose: Inception and progression of malignant melanoma is frequently associated with BRAF mutations in 35–45% of the cases. BRAF inhibitors (BRAFi) like vemurafenib (VF) may provide profound initial regression in melanoma with BRAF V600 mutations, but rapid resistance development often leads to enhanced metastasis and tumor invasion (Fig. 1). Aberrant regulation of an epigenetic reader like BRD4 (bromo domain protein 4) stands as a key driver in the development of resistance against BRAFi in melanoma. BRD4 targeted PROteolysis-TArgeting Chimera (PROTAC) (BPRO) selectively degrades the BRD4 protein and subsequently downregulates the c-Myc oncogene to enhance the cytotoxicity of VF and successfully overcome its resistance in melanoma. This is the very first study demonstrating oral nanoformulation development and characterization of PROTAC class of molecule. The objectives of our study were; (a) to identify the role of BPRO in overcoming VF resistance in 2D and 3D BRAFi-resistant melanoma models, (b) to develop and characterize BPRO and VF-loaded oral lipid nanocomplex (BV-LNC), and (c) to analyze the in vivo anticancer efficacy of BV-LNC in BRAFi-resistant tumor xenograft model.
Methods: Anti-proliferative effects of BPRO and VF were evaluated in parent (SK-MEL-28 and A375) and VF-resistant (SK-MEL-28R, A375R and RPMI-7951) melanoma cell lines using MTT cytotoxicity assay. In vitro migration and clonogenic assays were performed for BPRO, VF and their combination in resistant melanoma cells. Annexin V assay was used to determine apoptotic effect of BPRO and VF in resistant melanoma cell lines which was further analyzed using flow cytometry. To better mimic the in vivo tumor microenvironment, we evaluated the growth, apoptosis and invasion of 3D multicellular A375R spheroids following BPRO and VF treatment. BV-LNC incorporating BPRO (5% w/w) and VF (5% w/w) was developed after thorough optimization using ion-pairing approach followed by its physicochemical characterization in terms of particle size, polydispersity index and zeta potential. In vitro permeability assay was performed using Caco-2 cells to study the permeability characteristics of BPRO, VF and formulated BV-LNC. In vivo antitumor efficacy of B-LNC, V-LNC and BV-LNC was evaluated in VF-resistant (A375R) melanoma bearing athymic nude mice. Tumor volume and body weight were recorded, tumor growth kinetics was assessed, and survival study was performed for all the groups.
Results: Nanomolar IC50 values of BPRO obtained were found to be 5-6-fold lower in VF-resistant melanoma cells as compared to the parent melanoma cells (Fig. 2a). Further, in presence of non-cytotoxic concentrations of BPRO, cytotoxicity of VF was significantly enhanced to demonstrate their synergism (Fig. 2b). Combination of BPRO and VF also substantially inhibited the in vitro migration and clonogenic potential of resistant melanoma cell lines. Annexin V assay results demonstrated significant apoptotic effect mediated by this synergistic combination of BPRO and VF suggesting its strong antiproliferative activity in resistant melanoma cells (Fig. 2c). Similar combination of BPRO and VF led to significant reduction in the area and invasiveness of 3D resistant melanoma spheroids with large apoptotic population as depicted by their bright red fluorescence (Fig. 2d-f). Optimized BV-LNC spontaneously formed droplets with size of < 100 nm, neutral surface charge and unimodal size distribution to substantially enhance the solubility of BPRO (10-40-fold) and VF (200-300-fold) (Fig. 3a-b). BV-LNC significantly improved the in vitro permeability and oral bioavailability of both BPRO and VF by ~10 times (Fig. 3c). Most importantly, BV-LNC led to noteworthy in vivo tumor growth inhibition compared to individual treatment and control groups in VF-resistant melanoma xenograft model (Fig. 3d). The order of tumor growth rate of different groups was as BV-LNC < B-LNC < V-LNC < Control. Vascularization of BV-LNC treated tumor was substantially lower and led to significantly prolonged survival with 50% mice surviving until the experimental endpoint as compared to other groups (Fig. 3f,h). No significant differences in body weight were observed between treated and control animals (Fig. 3e). Intriguingly, treatment with BV-LNC cohort ensued lowest tumor growth rate, lowest T/C ratio, highest Tumor Growth Inhibition index and longest Absolute Growth Delay with negligible effect on WBC count as compared to other treatment or control groups (Fig. 3g). The tumor tissues are currently being analyzed for markers of proliferation, apoptosis, and angiogenesis including ki-67, caspase-3, CD-31, phospho-ERK and p-Akt using immunohistochemistry analysis.
Conclusion: Our research emphasizes that co-administration of BPRO and VF using an oral lipid nanocomplex successfully overcomes BRAFi-mediated resistance in melanoma. Promising in vivo antitumor activity and prolonged survival demonstrated by BV-LNC signifies its clinical translational potential as a novel therapeutic strategy for BRAFi-resistant melanoma.
Acknowledgements:This research was fully funded by the National Institutes of Health (NIH) grant [1SC2GM130478].
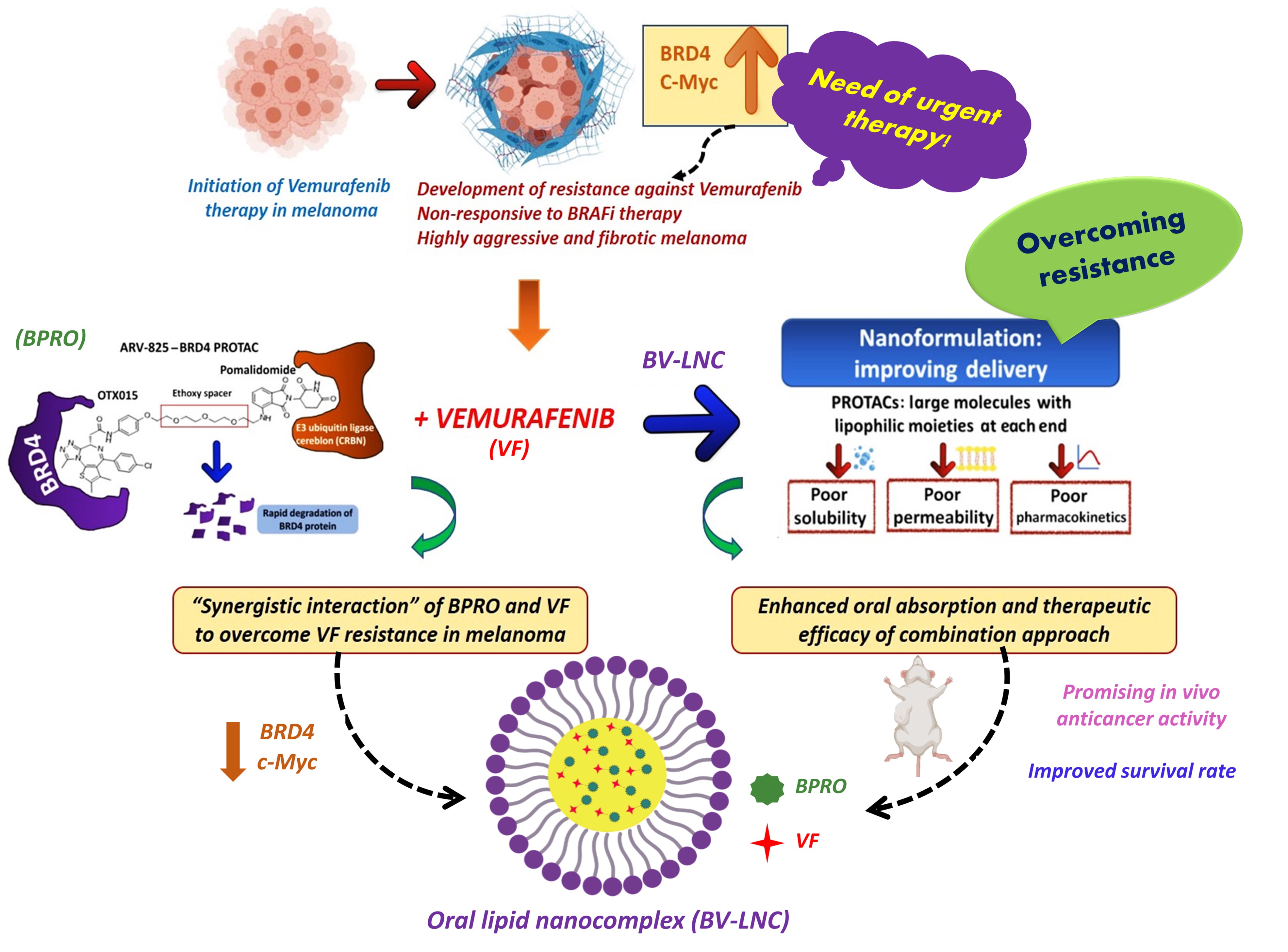
Fig. 1. An oral lipid nanocomplex of BPRO with VF (BV-LNC) as a potentially new combinatorial strategy to treat high aggressive and fibrotic BRAFi resistant malignant melanoma.
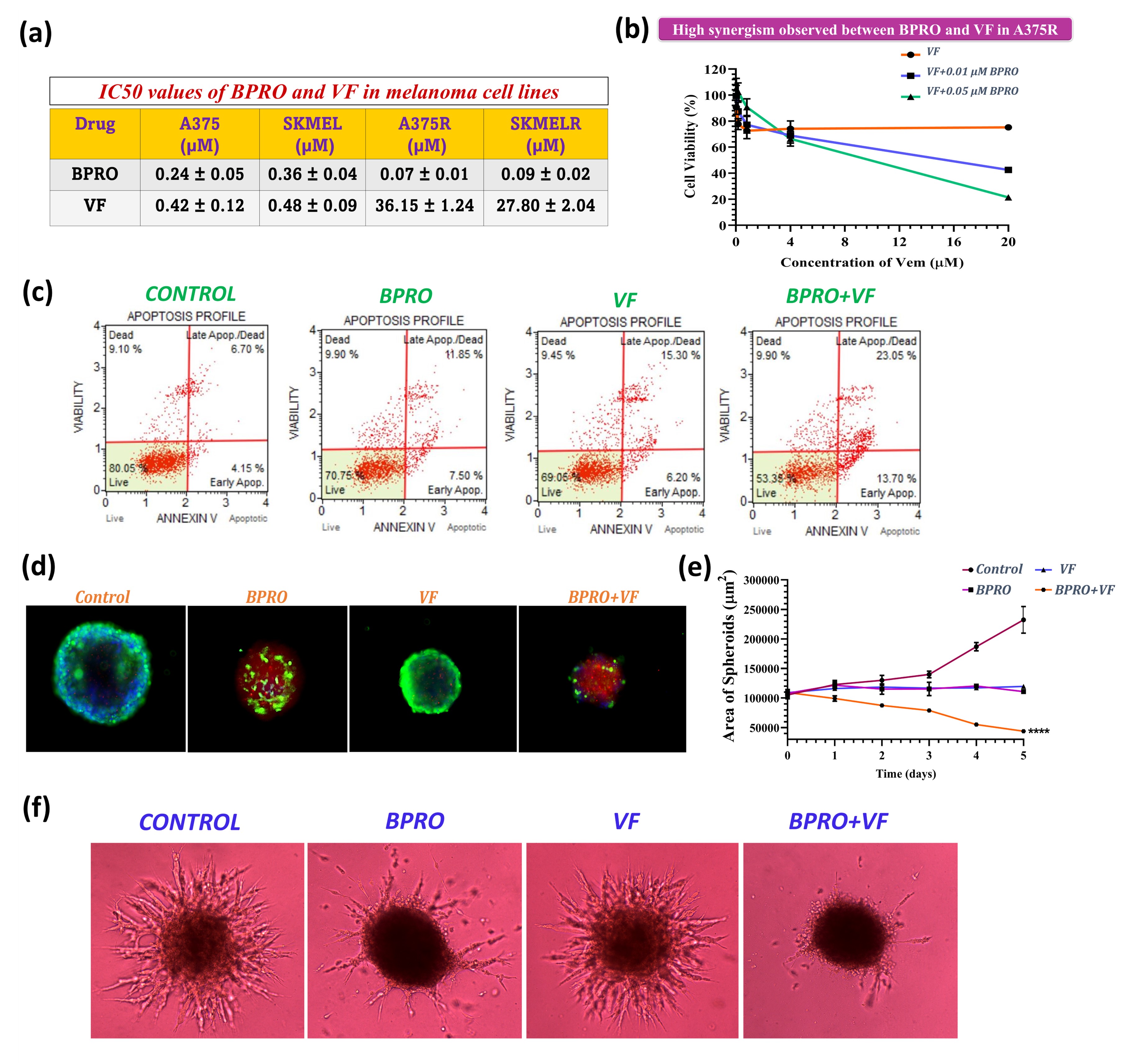
Fig. 2. (a) IC50 values of BPRO and VF in parent and VF-resistant melanoma cell lines. (b) Cytotoxicity of VF in combination with non-cytotoxic concentrations of BPRO in A375R cells. (c) Apoptosis assay results of BPRO and VF treated A375R cells. (d-e) Results of cell viability within BPRO and VF-treated 3D multicellular A375R spheroids. (f) BPRO and VF-treated 3D multicellular A375R spheroids depicting substantial inhibition in their invasiveness following 5 days of treatment. Scale bars, 400 μm. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
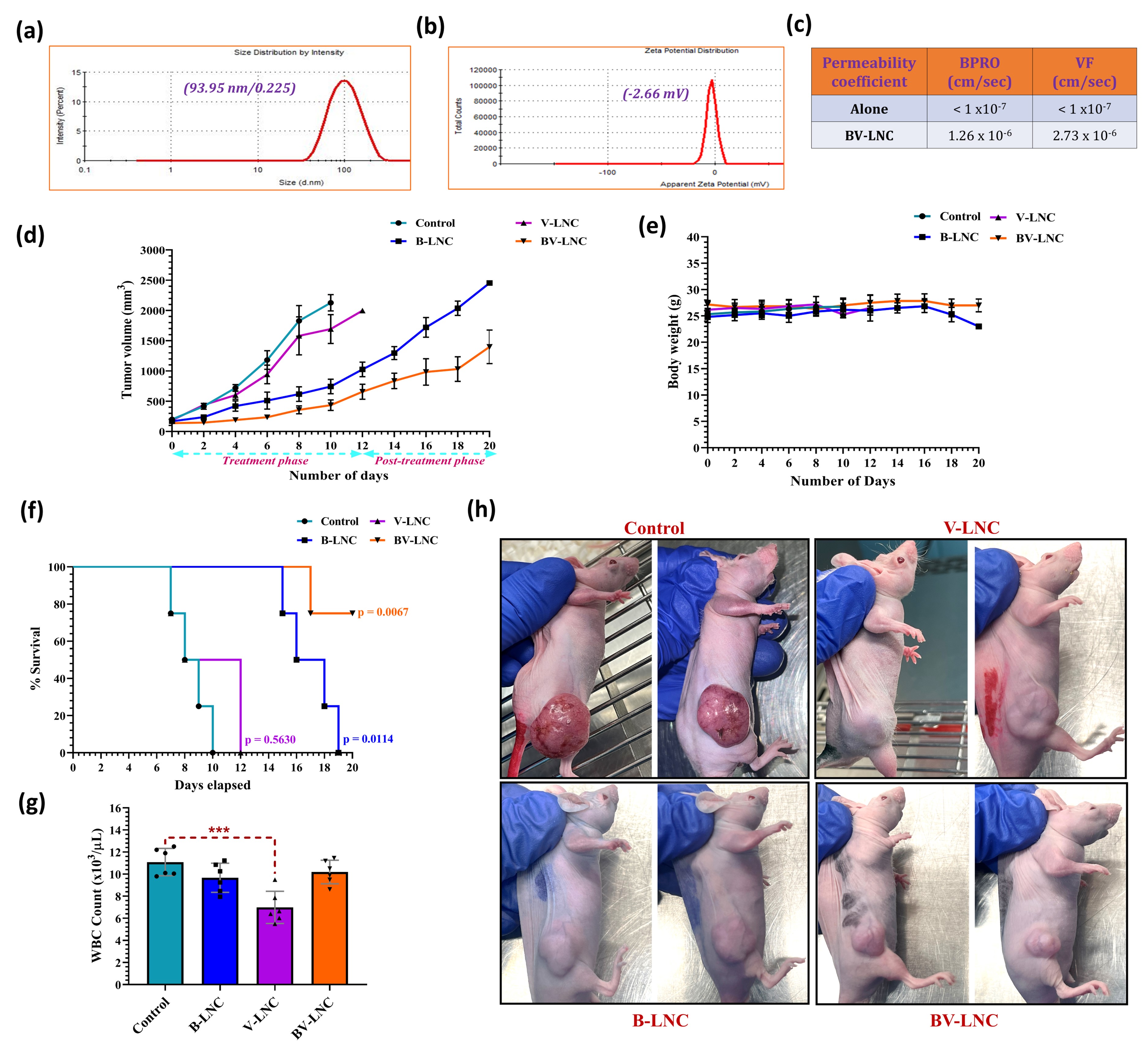
Fig. 3. (a-b) Physicochemical characterization and dynamic light scattering graphs illustrating monomodal size distribution and zeta potential of optimized BV-LNC. (c) In vitro permeability results for BV-LNC as compared to BPRO and VF alone. (d) Tumor growth curve of different treatment groups. (e) Effect of different treatment groups on body weight of mice. (f) Kaplan-Meier survival analysis for xenograft mice treated with various groups. (g) The average number of WBC in xenograft model after treatment with different groups. (h) Vascularization of tumors and anti-tumor effect after 10 days of treatment with different groups in BRAFi-resistant melanoma xenograft mice. Data are expressed as mean ± S.D. ***Statistically significant at p < 0.001.
Formulation and Delivery - Biomolecular - Formulation
Category: Poster Abstract
(T1330-07-40) An Oral Lipid Nanocomplex Incorporating BRD4 PROTAC and Vemurafenib Overcomes BRAF Inhibitor Resistance in Malignant Melanoma In Vivo
Tuesday, October 18, 2022
1:30 PM – 2:30 PM ET
- AS
Aishwarya Saraswat, MS
St. John's University
Jamaica, New York, United States - AS
Aishwarya Saraswat, MS
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Inception and progression of malignant melanoma is frequently associated with BRAF mutations in 35–45% of the cases. BRAF inhibitors (BRAFi) like vemurafenib (VF) may provide profound initial regression in melanoma with BRAF V600 mutations, but rapid resistance development often leads to enhanced metastasis and tumor invasion (Fig. 1). Aberrant regulation of an epigenetic reader like BRD4 (bromo domain protein 4) stands as a key driver in the development of resistance against BRAFi in melanoma. BRD4 targeted PROteolysis-TArgeting Chimera (PROTAC) (BPRO) selectively degrades the BRD4 protein and subsequently downregulates the c-Myc oncogene to enhance the cytotoxicity of VF and successfully overcome its resistance in melanoma. This is the very first study demonstrating oral nanoformulation development and characterization of PROTAC class of molecule. The objectives of our study were; (a) to identify the role of BPRO in overcoming VF resistance in 2D and 3D BRAFi-resistant melanoma models, (b) to develop and characterize BPRO and VF-loaded oral lipid nanocomplex (BV-LNC), and (c) to analyze the in vivo anticancer efficacy of BV-LNC in BRAFi-resistant tumor xenograft model.
Methods: Anti-proliferative effects of BPRO and VF were evaluated in parent (SK-MEL-28 and A375) and VF-resistant (SK-MEL-28R, A375R and RPMI-7951) melanoma cell lines using MTT cytotoxicity assay. In vitro migration and clonogenic assays were performed for BPRO, VF and their combination in resistant melanoma cells. Annexin V assay was used to determine apoptotic effect of BPRO and VF in resistant melanoma cell lines which was further analyzed using flow cytometry. To better mimic the in vivo tumor microenvironment, we evaluated the growth, apoptosis and invasion of 3D multicellular A375R spheroids following BPRO and VF treatment. BV-LNC incorporating BPRO (5% w/w) and VF (5% w/w) was developed after thorough optimization using ion-pairing approach followed by its physicochemical characterization in terms of particle size, polydispersity index and zeta potential. In vitro permeability assay was performed using Caco-2 cells to study the permeability characteristics of BPRO, VF and formulated BV-LNC. In vivo antitumor efficacy of B-LNC, V-LNC and BV-LNC was evaluated in VF-resistant (A375R) melanoma bearing athymic nude mice. Tumor volume and body weight were recorded, tumor growth kinetics was assessed, and survival study was performed for all the groups.
Results: Nanomolar IC50 values of BPRO obtained were found to be 5-6-fold lower in VF-resistant melanoma cells as compared to the parent melanoma cells (Fig. 2a). Further, in presence of non-cytotoxic concentrations of BPRO, cytotoxicity of VF was significantly enhanced to demonstrate their synergism (Fig. 2b). Combination of BPRO and VF also substantially inhibited the in vitro migration and clonogenic potential of resistant melanoma cell lines. Annexin V assay results demonstrated significant apoptotic effect mediated by this synergistic combination of BPRO and VF suggesting its strong antiproliferative activity in resistant melanoma cells (Fig. 2c). Similar combination of BPRO and VF led to significant reduction in the area and invasiveness of 3D resistant melanoma spheroids with large apoptotic population as depicted by their bright red fluorescence (Fig. 2d-f). Optimized BV-LNC spontaneously formed droplets with size of < 100 nm, neutral surface charge and unimodal size distribution to substantially enhance the solubility of BPRO (10-40-fold) and VF (200-300-fold) (Fig. 3a-b). BV-LNC significantly improved the in vitro permeability and oral bioavailability of both BPRO and VF by ~10 times (Fig. 3c). Most importantly, BV-LNC led to noteworthy in vivo tumor growth inhibition compared to individual treatment and control groups in VF-resistant melanoma xenograft model (Fig. 3d). The order of tumor growth rate of different groups was as BV-LNC < B-LNC < V-LNC < Control. Vascularization of BV-LNC treated tumor was substantially lower and led to significantly prolonged survival with 50% mice surviving until the experimental endpoint as compared to other groups (Fig. 3f,h). No significant differences in body weight were observed between treated and control animals (Fig. 3e). Intriguingly, treatment with BV-LNC cohort ensued lowest tumor growth rate, lowest T/C ratio, highest Tumor Growth Inhibition index and longest Absolute Growth Delay with negligible effect on WBC count as compared to other treatment or control groups (Fig. 3g). The tumor tissues are currently being analyzed for markers of proliferation, apoptosis, and angiogenesis including ki-67, caspase-3, CD-31, phospho-ERK and p-Akt using immunohistochemistry analysis.
Conclusion: Our research emphasizes that co-administration of BPRO and VF using an oral lipid nanocomplex successfully overcomes BRAFi-mediated resistance in melanoma. Promising in vivo antitumor activity and prolonged survival demonstrated by BV-LNC signifies its clinical translational potential as a novel therapeutic strategy for BRAFi-resistant melanoma.
Acknowledgements:This research was fully funded by the National Institutes of Health (NIH) grant [1SC2GM130478].

Fig. 1. An oral lipid nanocomplex of BPRO with VF (BV-LNC) as a potentially new combinatorial strategy to treat high aggressive and fibrotic BRAFi resistant malignant melanoma.

Fig. 2. (a) IC50 values of BPRO and VF in parent and VF-resistant melanoma cell lines. (b) Cytotoxicity of VF in combination with non-cytotoxic concentrations of BPRO in A375R cells. (c) Apoptosis assay results of BPRO and VF treated A375R cells. (d-e) Results of cell viability within BPRO and VF-treated 3D multicellular A375R spheroids. (f) BPRO and VF-treated 3D multicellular A375R spheroids depicting substantial inhibition in their invasiveness following 5 days of treatment. Scale bars, 400 μm. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.

Fig. 3. (a-b) Physicochemical characterization and dynamic light scattering graphs illustrating monomodal size distribution and zeta potential of optimized BV-LNC. (c) In vitro permeability results for BV-LNC as compared to BPRO and VF alone. (d) Tumor growth curve of different treatment groups. (e) Effect of different treatment groups on body weight of mice. (f) Kaplan-Meier survival analysis for xenograft mice treated with various groups. (g) The average number of WBC in xenograft model after treatment with different groups. (h) Vascularization of tumors and anti-tumor effect after 10 days of treatment with different groups in BRAFi-resistant melanoma xenograft mice. Data are expressed as mean ± S.D. ***Statistically significant at p < 0.001.
