Back
Purpose: Poor aqueous solubility of new chemical entities is a well-known and systematically studied challenge in the development of oral drug products. The probably most investigated approach to tackle this challenge is the formulation as an amorphous solid dispersion (ASD). In this concept the drug is molecularly dispersed in a polymeric carrier, which supports the spring (quick supersaturation) and parachute (prolonged recrystallisation inhibition) effect (long recrystallisation inhibition) during dissolution. Even though polymethacrylates, celluloses and povidones have been proven to be the most successful polymeric carriers [1], still there is no “one fits all”-solution available to overcome solubility challenges for all poorly soluble APIs. In addition, processing issues can lead to long processing times which have an impact on the final dosage form cost [2]. In this study the synergistic effect of polymethacrylate and cellulose carriers [3] have been evaluated.
Methods: Physical mixtures and co-processed excipients of EUDRAGIT® E PO and EUDRAGIT® L 100-55 with NISSO HPC-SSL in different ratios were prepared via blending and organic spray drying, respectively. A possible formation of covalent bonds was evaluated via NMR and IR analysis. The miscibility of the polymers was investigated via DSC measurements. ASDs with the model drugs celecoxib, fenofibrate and clotrimazole were prepared via spray drying and HME in order to evaluate possible benefits in the processing and solubility enhancing performance of these combined carriers.
Results: No new covalent bonds were detected after the co-spray drying of EUDRAGIT® E PO and EUDRAGIT® L100-55 with HPC-SSL in NMR and IR analyses. The DSC of all mixtures revealed a single Tg during the second heating that speaks for good miscibility of the polymers and low risk of amorphous phase separation in solid state. ASDs with celecoxib and EUDRAGIT® L 100-55/HPC-SSL (50:50 physical mixture and co-processed excipient) in 1:1 and 1:5 API:polymer ratios prepared via organic spray drying showed a 100% increase in dissolution compared to the crystalline API and a higher dissolution compared to EUDRAGIT® L 100-55 as a single carrier. The solid content of the spray solution could be increased from 6% with the single polymeric carrier to 10-15% for the physical mixture and to 15% for the co-processed excipient. The same increased solid content of the spray solution could be achieved for combinations with clotrimazole. ASDs composed of fenofibrate and EUDRAGIT® E/HPC-SSL prepared via HME exhibited good physical properties and could overcome rubbery state of combinations with EUDRAGIT® E PO alone.
Conclusion: The data showed that combining EUDRAGIT® with HPC-SSL could improve the solubility enhancing performance compared to a single polymeric carrier. In addition, the physical habit of the resulting strands and powders could be improved. In terms of processing via spray drying the physical mixing of both polymers resulted in a higher spray solution concentration with an even increased solid content for the co-processed excipient. This study proves the synergistic benefits of combining polymeric carriers to fully leverage the solubility enhancing potential and maximize economic output.
References: [1] S. Bhujbal et al., Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies, Acta Pharmaceutica Sinica B, 11(8), 2505-2536, 2021
[2] K. Shepard et al., Impact of process parameters on particle morphology and filament formation in spray dried Eudragit L100 polymer, Powder Technology 362(2), 2019
[3] C. Luebbert et al., Phase behavior of ASDs based on hydroxypropyl cellulose, International Journal of Pharmaceutics X3, 2021,100070
Acknowledgements:
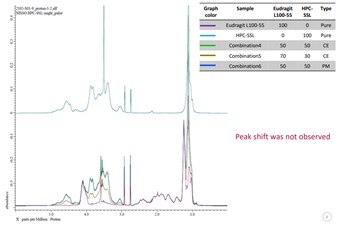
Figure 1: 1H-NMR for EUDRAGIT® L 100-55 (purple) / HPC-SSL (blue); single components, physical mixture (dark blue) and co-processed excipients (green 50:50, brown 70:30)
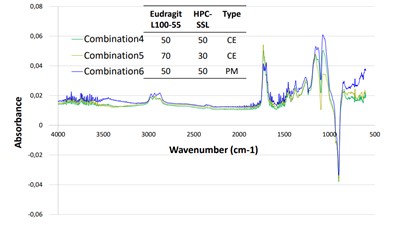
Figure 2: IR-ATR for EUDRAGIT® L 100-55 / HPC-SSL physical mixture (dark blue) and co-processed excipients (green 50:50, brown 70:30)
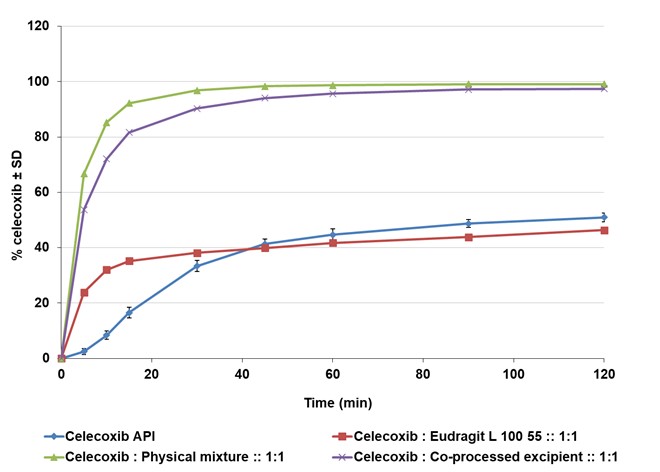
Figure 3: Dissolution profile of celecoxib crystalline drug and ASDs in phosphate buffer pH 6.8 with 0.2% tween 80
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T0930-03-16) Investigating the Synergistic Effect of Polymethacrylates and HPC as Polymeric Carrier for Drug Solubility Enhancement
Tuesday, October 18, 2022
9:30 AM – 10:30 AM ET

Santhosh Punnoose, MS
Principal Scientist
Evonik Corporation
Piscataway, New Jersey, United States- PH
Priyanka Haksar, MS
Evonik India Pvt., Ltd.
Mumbai, Maharashtra, India
Presenter (non-author)(s)
Main Author(s)
Purpose: Poor aqueous solubility of new chemical entities is a well-known and systematically studied challenge in the development of oral drug products. The probably most investigated approach to tackle this challenge is the formulation as an amorphous solid dispersion (ASD). In this concept the drug is molecularly dispersed in a polymeric carrier, which supports the spring (quick supersaturation) and parachute (prolonged recrystallisation inhibition) effect (long recrystallisation inhibition) during dissolution. Even though polymethacrylates, celluloses and povidones have been proven to be the most successful polymeric carriers [1], still there is no “one fits all”-solution available to overcome solubility challenges for all poorly soluble APIs. In addition, processing issues can lead to long processing times which have an impact on the final dosage form cost [2]. In this study the synergistic effect of polymethacrylate and cellulose carriers [3] have been evaluated.
Methods: Physical mixtures and co-processed excipients of EUDRAGIT® E PO and EUDRAGIT® L 100-55 with NISSO HPC-SSL in different ratios were prepared via blending and organic spray drying, respectively. A possible formation of covalent bonds was evaluated via NMR and IR analysis. The miscibility of the polymers was investigated via DSC measurements. ASDs with the model drugs celecoxib, fenofibrate and clotrimazole were prepared via spray drying and HME in order to evaluate possible benefits in the processing and solubility enhancing performance of these combined carriers.
Results: No new covalent bonds were detected after the co-spray drying of EUDRAGIT® E PO and EUDRAGIT® L100-55 with HPC-SSL in NMR and IR analyses. The DSC of all mixtures revealed a single Tg during the second heating that speaks for good miscibility of the polymers and low risk of amorphous phase separation in solid state. ASDs with celecoxib and EUDRAGIT® L 100-55/HPC-SSL (50:50 physical mixture and co-processed excipient) in 1:1 and 1:5 API:polymer ratios prepared via organic spray drying showed a 100% increase in dissolution compared to the crystalline API and a higher dissolution compared to EUDRAGIT® L 100-55 as a single carrier. The solid content of the spray solution could be increased from 6% with the single polymeric carrier to 10-15% for the physical mixture and to 15% for the co-processed excipient. The same increased solid content of the spray solution could be achieved for combinations with clotrimazole. ASDs composed of fenofibrate and EUDRAGIT® E/HPC-SSL prepared via HME exhibited good physical properties and could overcome rubbery state of combinations with EUDRAGIT® E PO alone.
Conclusion: The data showed that combining EUDRAGIT® with HPC-SSL could improve the solubility enhancing performance compared to a single polymeric carrier. In addition, the physical habit of the resulting strands and powders could be improved. In terms of processing via spray drying the physical mixing of both polymers resulted in a higher spray solution concentration with an even increased solid content for the co-processed excipient. This study proves the synergistic benefits of combining polymeric carriers to fully leverage the solubility enhancing potential and maximize economic output.
References: [1] S. Bhujbal et al., Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies, Acta Pharmaceutica Sinica B, 11(8), 2505-2536, 2021
[2] K. Shepard et al., Impact of process parameters on particle morphology and filament formation in spray dried Eudragit L100 polymer, Powder Technology 362(2), 2019
[3] C. Luebbert et al., Phase behavior of ASDs based on hydroxypropyl cellulose, International Journal of Pharmaceutics X3, 2021,100070
Acknowledgements:

Figure 1: 1H-NMR for EUDRAGIT® L 100-55 (purple) / HPC-SSL (blue); single components, physical mixture (dark blue) and co-processed excipients (green 50:50, brown 70:30)

Figure 2: IR-ATR for EUDRAGIT® L 100-55 / HPC-SSL physical mixture (dark blue) and co-processed excipients (green 50:50, brown 70:30)

Figure 3: Dissolution profile of celecoxib crystalline drug and ASDs in phosphate buffer pH 6.8 with 0.2% tween 80
