Back
Purpose: WLBU2 (RRWVRRVRRWVRRVVRVVRRWVRR, 3400.12 Da) is an engineered antimicrobial peptide (AMP) with an amphipathic and cationic structure given by the repetition of only three aminoacids, i.e. Valine, Tryptophan, and Arginine. Acting as a bacterial membrane de-stabilizer, WLBU2 at 0.05 mg/kg i.t. was reported to induce a rapid decrease of P. aeruginosa burden in mice, showing its antimicrobial activity against different clinical isolates, including the ones from CF patients. Nevertheless, there is the need to delivery intact WLBU2 in conductive airways shielding its interactions with mucus or bacterial biofilm. On these considerations, the aims of this work were the design, the development, and the characterization of spray dried microparticles of WLBU2 for pulmonary delivery as dry powder.
Methods: A full factorial Design of Experiments (23) was followed for the preparation of spray-dried microparticles of WLBU2 antimicrobial peptide. Aqueous solution of WLBU2 at 20% w/w with Mannitol (60% w/w) as inert carrier and leucine (20% w/w) as anti-hygroscopic agent were dried keeping constant the formulation composition. The feed rate (3-5 ml/min), the inlet temperature (90-120°C) and the feed concentration (1-5 mg/ml) were the Critical Process Parameters selected and varied during the Design of Experiments (DoE). The peptide content (%) within the microparticles was assessed by HPLC after analytical method development. In the context of the Quality by Design, and as stated by the DoE, powders were characterized for four Critical Quality Attributes: the yield of the drying (%); the particle’s size (µm) assessed by Laser diffraction; the aerodynamic performance, carried out using the Fast Screening Impactor, expressed considering the Emitted Dose (mg) and the Fine Particle Fraction (%); the moisture content (%) determined by Thermogravimetric analysis. Finally, the morphology of the microparticles, which is another important parameter affecting the aerosolization behavior, was observed by Scanning Electron Microscopy.
Results: The feed rate during the drying step and the feed concentration were the dominant factors affected the Critical Quality Attributes selected for the dried WLBU2 microparticles. These factors had positive and negative effects on the responses and focusing on the CQAs determining the respirability of the powders, i.e the size (µm), the ED (mg) and the FPF (%), different behaviors were observed. Smaller particles were obtained at lower values of feed concentration since at high concentration more energy is required to atomize the solution. These microparticles resulted in a Dv50 of 3.7± 0.38 µm, suitable for pulmonary delivery, with the highest FPF value of 83.82%. FPF is an indicator of the deposition behaviour of particles in the lung and high values were obtained at low feed concentration. For the emitted dose, a non-linear mathematical relationship between factors and response was observed. As consequence, a design augmentation was needed. The morphology of the microparticles was observed by Scanning Electron Microscopy. SEM images revealed quite spherical particles, slightly wrinkled due to the leucine. Indeed, Leucine added at 10-20% w/w of the total solid content in the solution to be dried, was reported to cover the microparticles, wrinkling and protecting them from moisture. Moreover, this morphology was quite in agreement with the requirement of the pulmonary administration, and this may contribute to the explanation of the good FPF values obtained for many formulations.
Conclusion: The spray drying process was suitable for WLBU2 dry powder preparation, also because a quantitative analysis of the drug content within the powder revealed a peptide content of about 98%±1.74%. The two-level factorial design was kept as simple as possible to screen variables during the process development. All the statistically significant effects and interactions were mapped evidencing the predominant effect of the feed concentration and the feed rate in determining the quality of the microparticles. This agreed with previous literature statement where the solid content was reported to affect the aerodynamic behavior of particles after solvent evaporation as well as the feed rate was reported to have relevance for the industrial manufacturing time.
References: 1. Chen C., Clinical Microbiology and Infection, 2018
2. Politis N.P., Rekkas M.D., Drug deliveri and Industrial Pharmacy, 2017.
3. Tavares Luiz M, Eur J. Pharm and Biopharm, 2021.
4. Malamatari M, Processes, 2020.
5. Ordoubadi et al., Int J Pharm, 2021;
6. Winston Duo Wu, Journal of Food Engineering, 2014.
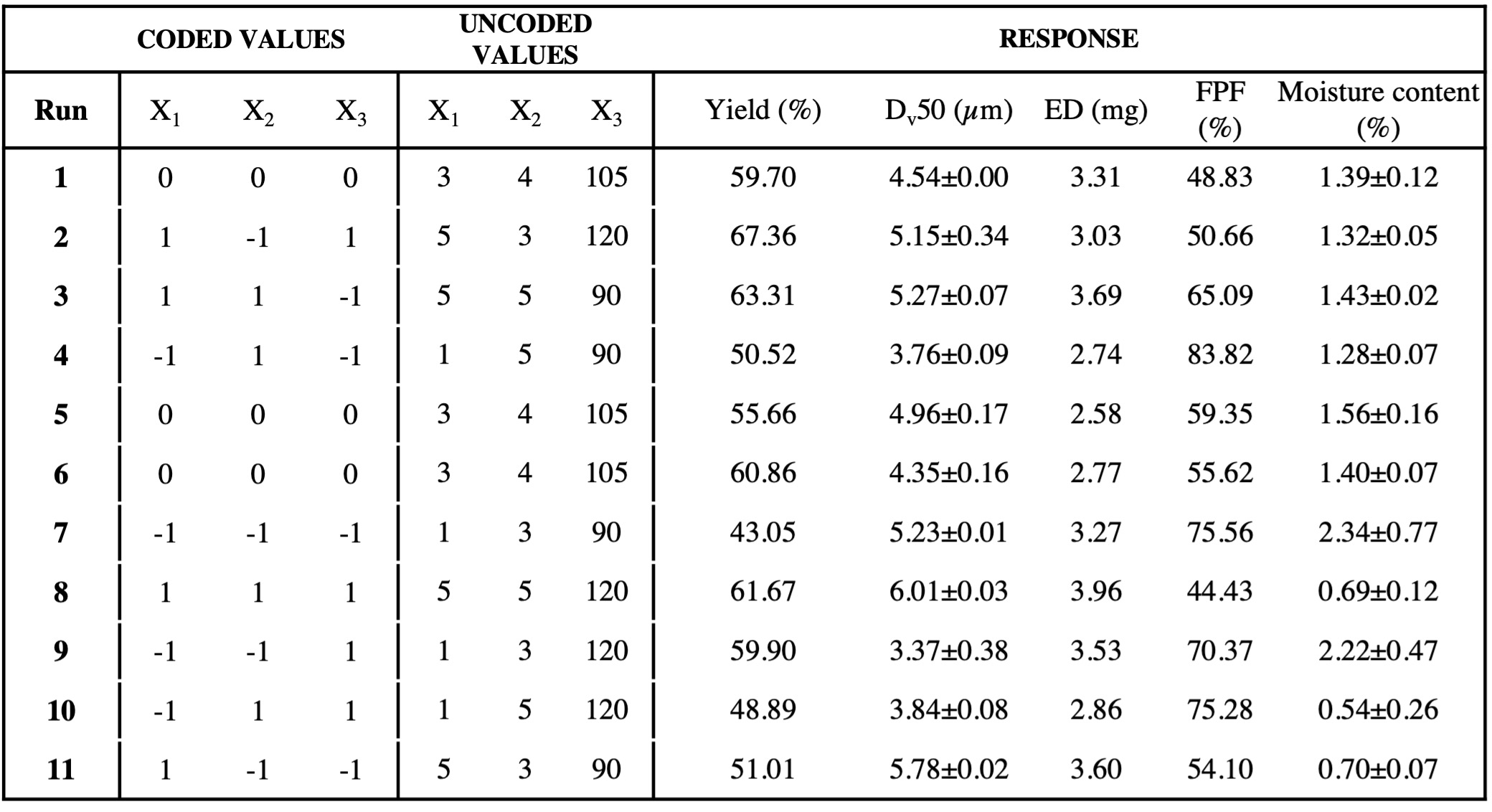
Matrix of the Full Factorial Design created and followed for powders manufacturing.
.jpg)
Scanning Electron Microscopy image of WLBU2 spray dried microparticles.
Formulation and Delivery - Biomolecular - Administration
Category: Poster Abstract
(M1530-01-04) Formulation Design of WLBU2 Antimicrobial Peptide Dry Powder for Lung Infections
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- AR
Alessandra Rossi
University of Parma
Parma, Emilia-Romagna, Italy - CO
Chiara Ogliari
PhD Student
The University of Parma
Parma, Emilia-Romagna, Italy
Presenting Author(s)
Main Author(s)
Purpose: WLBU2 (RRWVRRVRRWVRRVVRVVRRWVRR, 3400.12 Da) is an engineered antimicrobial peptide (AMP) with an amphipathic and cationic structure given by the repetition of only three aminoacids, i.e. Valine, Tryptophan, and Arginine. Acting as a bacterial membrane de-stabilizer, WLBU2 at 0.05 mg/kg i.t. was reported to induce a rapid decrease of P. aeruginosa burden in mice, showing its antimicrobial activity against different clinical isolates, including the ones from CF patients. Nevertheless, there is the need to delivery intact WLBU2 in conductive airways shielding its interactions with mucus or bacterial biofilm. On these considerations, the aims of this work were the design, the development, and the characterization of spray dried microparticles of WLBU2 for pulmonary delivery as dry powder.
Methods: A full factorial Design of Experiments (23) was followed for the preparation of spray-dried microparticles of WLBU2 antimicrobial peptide. Aqueous solution of WLBU2 at 20% w/w with Mannitol (60% w/w) as inert carrier and leucine (20% w/w) as anti-hygroscopic agent were dried keeping constant the formulation composition. The feed rate (3-5 ml/min), the inlet temperature (90-120°C) and the feed concentration (1-5 mg/ml) were the Critical Process Parameters selected and varied during the Design of Experiments (DoE). The peptide content (%) within the microparticles was assessed by HPLC after analytical method development. In the context of the Quality by Design, and as stated by the DoE, powders were characterized for four Critical Quality Attributes: the yield of the drying (%); the particle’s size (µm) assessed by Laser diffraction; the aerodynamic performance, carried out using the Fast Screening Impactor, expressed considering the Emitted Dose (mg) and the Fine Particle Fraction (%); the moisture content (%) determined by Thermogravimetric analysis. Finally, the morphology of the microparticles, which is another important parameter affecting the aerosolization behavior, was observed by Scanning Electron Microscopy.
Results: The feed rate during the drying step and the feed concentration were the dominant factors affected the Critical Quality Attributes selected for the dried WLBU2 microparticles. These factors had positive and negative effects on the responses and focusing on the CQAs determining the respirability of the powders, i.e the size (µm), the ED (mg) and the FPF (%), different behaviors were observed. Smaller particles were obtained at lower values of feed concentration since at high concentration more energy is required to atomize the solution. These microparticles resulted in a Dv50 of 3.7± 0.38 µm, suitable for pulmonary delivery, with the highest FPF value of 83.82%. FPF is an indicator of the deposition behaviour of particles in the lung and high values were obtained at low feed concentration. For the emitted dose, a non-linear mathematical relationship between factors and response was observed. As consequence, a design augmentation was needed. The morphology of the microparticles was observed by Scanning Electron Microscopy. SEM images revealed quite spherical particles, slightly wrinkled due to the leucine. Indeed, Leucine added at 10-20% w/w of the total solid content in the solution to be dried, was reported to cover the microparticles, wrinkling and protecting them from moisture. Moreover, this morphology was quite in agreement with the requirement of the pulmonary administration, and this may contribute to the explanation of the good FPF values obtained for many formulations.
Conclusion: The spray drying process was suitable for WLBU2 dry powder preparation, also because a quantitative analysis of the drug content within the powder revealed a peptide content of about 98%±1.74%. The two-level factorial design was kept as simple as possible to screen variables during the process development. All the statistically significant effects and interactions were mapped evidencing the predominant effect of the feed concentration and the feed rate in determining the quality of the microparticles. This agreed with previous literature statement where the solid content was reported to affect the aerodynamic behavior of particles after solvent evaporation as well as the feed rate was reported to have relevance for the industrial manufacturing time.
References: 1. Chen C., Clinical Microbiology and Infection, 2018
2. Politis N.P., Rekkas M.D., Drug deliveri and Industrial Pharmacy, 2017.
3. Tavares Luiz M, Eur J. Pharm and Biopharm, 2021.
4. Malamatari M, Processes, 2020.
5. Ordoubadi et al., Int J Pharm, 2021;
6. Winston Duo Wu, Journal of Food Engineering, 2014.

Matrix of the Full Factorial Design created and followed for powders manufacturing.
.jpg)
Scanning Electron Microscopy image of WLBU2 spray dried microparticles.
