Back
Purpose: Layer-by-Layer (LbL) assembly of polyelectrolytes on a solid particle is a well-established technique, that has been used for delivering drugs, proteins, regenerative medicines, genes, combinatorial therapies, etc. Despite all these advantages, its potential in non-invasive pulmonary delivery remains unexplored. Therefore, in the current study, we have assembled nanostructured thin films on microscopic particles of different sizes to form LbL coated particles for inhalation and have studied their therapeutic efficacy in small cell lung cancer (SCLC).
Methods: Layer-by-Layer assembly were prepared using two sets of polyelectrolytes, Chitosan and Bovine serum albumin (BSA). The polyelectrolytes were coated on 0.5- and 4.5-µm polystyrene particles in succession, thus forming 3 bilayers. The prepared LbL coated particles were characterized for particle size using light scattering analysis in comparison to the plain polystyrene particles. Zeta potential was measured to confirm the formation of polyelectrolyte coating after each layer. Further, the morphology of the LbL coated particles was studied in comparison to the plain particles using a scanning electron microscope (SEM). Thereafter, the LbL coated particles were analyzed for in-vitro aerosolization using Next Generation Impactor (NGI) with two separate nebulizers, Pari LC Plus and AeroEclipse XL. To check the therapeutic efficacy of these particles against SCLC, Doxorubicin (Dox) was passively loaded into the layers of the LbL coated particle by incubating in the drug solution for 12 hours. The Dox-loaded LbL particles were then collected by centrifugation and washed 3 times using DI water. The collected particles were analyzed for drug content and in-vitro drug release in PBS. Next, the therapeutic efficacy of the dox-loaded LbL particles was studied in multidrug-resistant H69AR SCLC cells by performing an in-vitro cytotoxicity assessment. Anti-tumor efficacy of Dox-loaded LbL particles was tested in an in-vitro 3D spheroid model.
Results: The light scattering analysis for Layer-by-Layer coated particles demonstrated a sequential increase in the particle size after the accumulation of each bilayer which confirmed the effective deposition of polyelectrolytes on both the particles 0.5-µm and 4.5-µm. This was further confirmed with the reversible zeta potential of the particles after deposition of each layer. The SEM images showed the morphology of LbL coated particles covered in thin layers constructed around the polystyrene particles with an uneven surface as compared to plain polystyrene particles having a relatively smooth surface (Figure 1). The in-vitro aerosolization study showed both 0.5-µm and 4.5-µm LbL particles to be inhalable with the mass median aerodynamic diameter (MMAD) of 3.6±0.1 µm and 4.2±0.7 µm with Pari LC nebulizer and 4.2±0.2 µm and 4.5±0.0 µm with AeroEclipse nebulizer, respectively, and more than 70% fine particle fraction (FPF) for both the nebulizers. We were able to passively load 0.07 mg/mg of Dox into the 0.5-µm LbL particles whereas 0.06 mg/mg in 4.5-µm LbL particles. The in-vitro release studies presented a sustained release for both the fabricated particles with ~80% and ~60% drug release from 0.5 and 4.5-µm LbL coated particles in 72 hours (Figure 2). However, the cytotoxicity assessment in Dox resistant H69AR cells demonstrated 0.5-µm LbL particles to be more efficient than 4.5-µm LbL particles with IC50 (0.5-µm) ≤ IC50 (plain dox) ≤ IC50 (4.5-µm) (Figure 3). Therefore, physiologically more relevant in-vitro tumor simulation, 3D-spheroid study was performed for 0.5-µm LbL coated particles and dox at equivalent concentrations of 3.125 and 6.25 µM over a period of 15 days.
Conclusion: The polystyrene particles were successfully coated with successive layers of Chitosan/BSA polyelectrolytes. The deposition was confirmed by increase in the effective diameter and reversible zeta potential of the particles after addition of layers. The SEM images showed the change in surface morphology of the particles on coating with polyelectrolytes. The NGI study showed LbL coated particles are inhalable. Sustained drug release was achieved for the fabricated LbL coated particles. The in-vitro efficacy of the 0.5-µm LbL particles against SCLC cells was achieved which will further be confirmed in an in-vitro tumor simulation study using 3D tumor model mimicking the in-vivo tumorigenic conditions.
Acknowledgements: The authors would like to acknowledge the Imaging Facility of CUNY Advanced Science Research Center for instrument use scientific and technical assistance.
Funding: This project was funded with start-up funds to Vivek Gupta by the College of Pharmacy and Health Sciences, St. John's University, Queens, NY. Gautam Chauhan was supported with the teaching assistantships by St. John's University!
Conflict of Interest: All authors declare no conflict of interest.
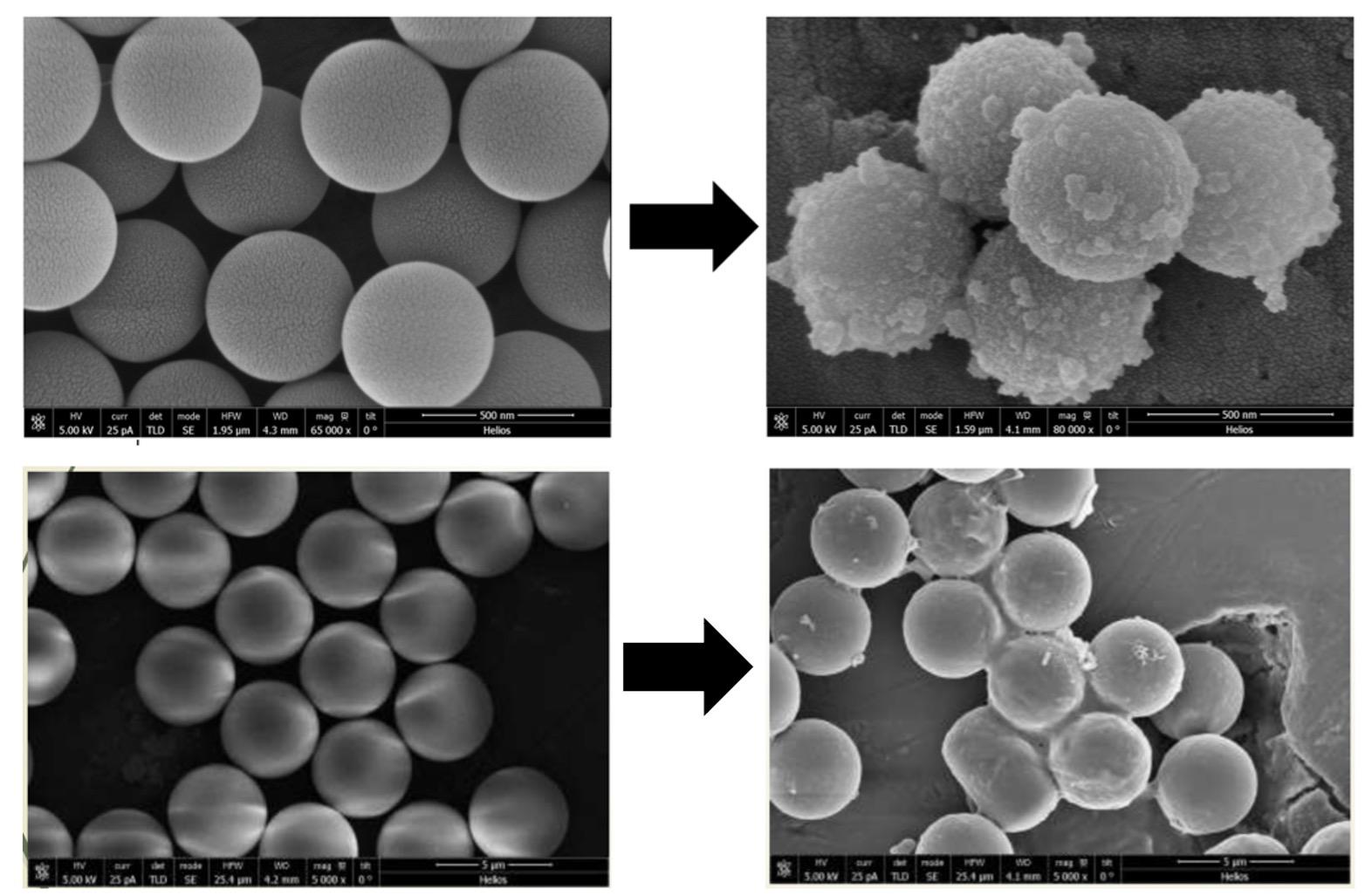
Figure 1: SEM images of LbL coated Polystyrene particles before and after deposition of 6 polyelectrolyte layers. The resultant particles preserved a spherical shape and are uniform in size.
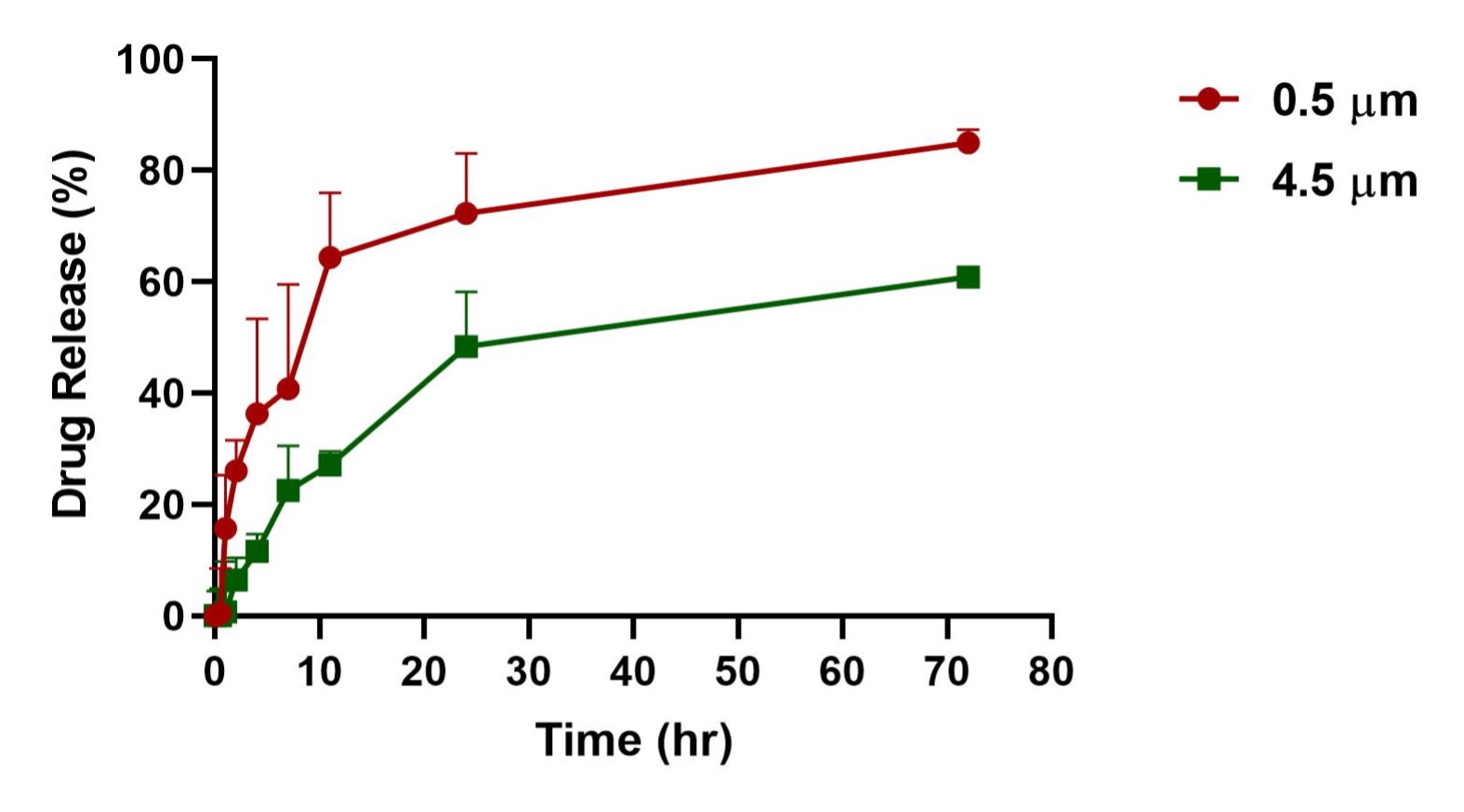
Figure 2: In-vitro release profile of Doxorubicin-loaded LbL coated 0.5- and 4.5-µm particles over a period of 3 days. Data represent mean ± SD (n = 3)
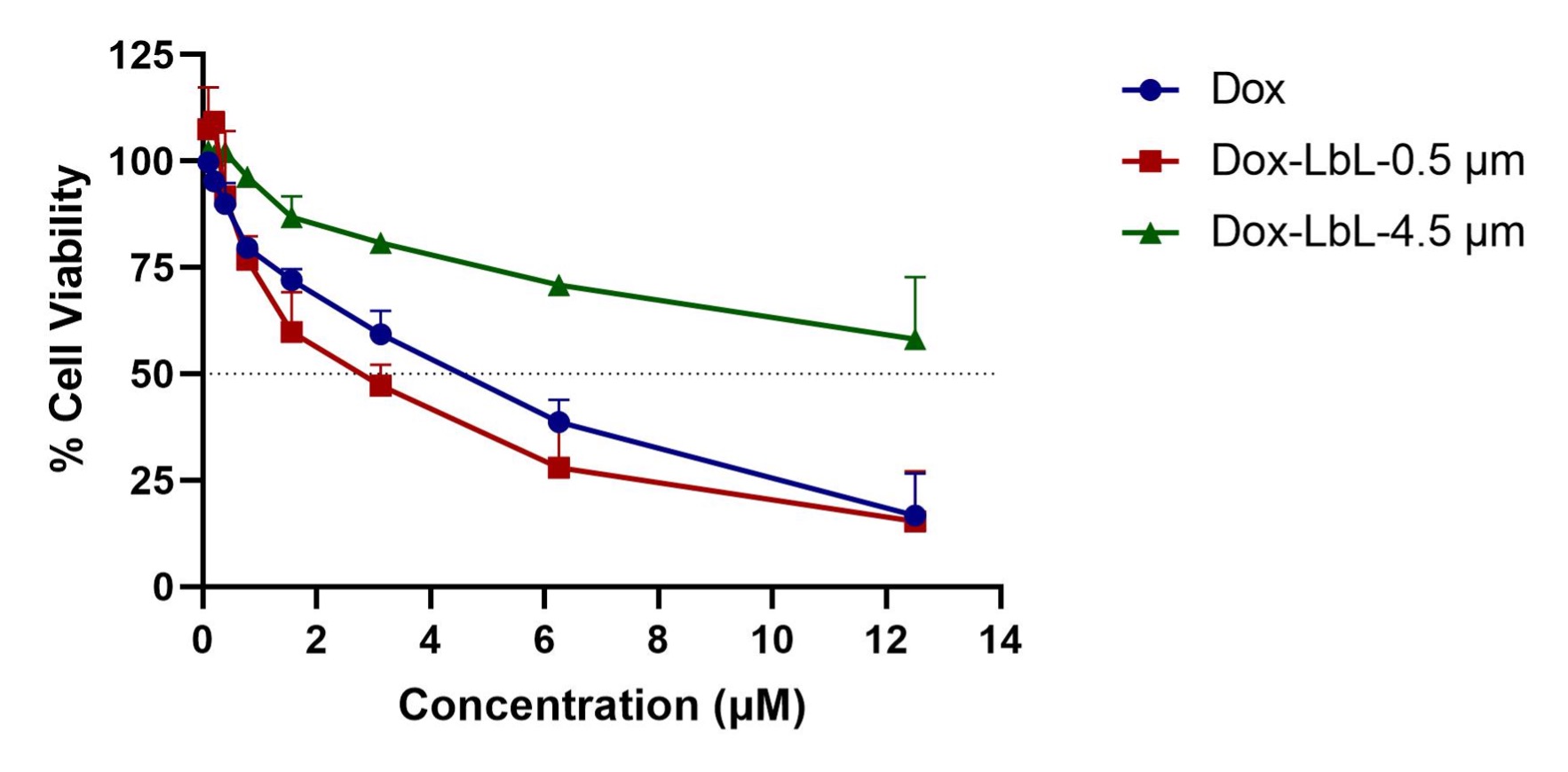
Figure 3: Cytotoxicity profile of Doxorubicin, Doxorubicin loaded LbL coated 0.5- and 4.5-µm particles at equivalent Dox concentration in small cell lung cancer (SCLC) cell line H69AR after 72 h of treatment. Data represent the mean ± SD of 2 individual experiments with n = 6 for each experiment.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1430-07-37) Development of Inhalable Layer-by-Layer Assembled Particles for Small Cell Lung Cancer (SCLC) Treatment
Monday, October 17, 2022
2:30 PM – 3:30 PM ET
- GC
Gautam Chauhan, M.S.
St. John's University
Jamaica, New York, United States - GC
Gautam Chauhan, M.S.
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Layer-by-Layer (LbL) assembly of polyelectrolytes on a solid particle is a well-established technique, that has been used for delivering drugs, proteins, regenerative medicines, genes, combinatorial therapies, etc. Despite all these advantages, its potential in non-invasive pulmonary delivery remains unexplored. Therefore, in the current study, we have assembled nanostructured thin films on microscopic particles of different sizes to form LbL coated particles for inhalation and have studied their therapeutic efficacy in small cell lung cancer (SCLC).
Methods: Layer-by-Layer assembly were prepared using two sets of polyelectrolytes, Chitosan and Bovine serum albumin (BSA). The polyelectrolytes were coated on 0.5- and 4.5-µm polystyrene particles in succession, thus forming 3 bilayers. The prepared LbL coated particles were characterized for particle size using light scattering analysis in comparison to the plain polystyrene particles. Zeta potential was measured to confirm the formation of polyelectrolyte coating after each layer. Further, the morphology of the LbL coated particles was studied in comparison to the plain particles using a scanning electron microscope (SEM). Thereafter, the LbL coated particles were analyzed for in-vitro aerosolization using Next Generation Impactor (NGI) with two separate nebulizers, Pari LC Plus and AeroEclipse XL. To check the therapeutic efficacy of these particles against SCLC, Doxorubicin (Dox) was passively loaded into the layers of the LbL coated particle by incubating in the drug solution for 12 hours. The Dox-loaded LbL particles were then collected by centrifugation and washed 3 times using DI water. The collected particles were analyzed for drug content and in-vitro drug release in PBS. Next, the therapeutic efficacy of the dox-loaded LbL particles was studied in multidrug-resistant H69AR SCLC cells by performing an in-vitro cytotoxicity assessment. Anti-tumor efficacy of Dox-loaded LbL particles was tested in an in-vitro 3D spheroid model.
Results: The light scattering analysis for Layer-by-Layer coated particles demonstrated a sequential increase in the particle size after the accumulation of each bilayer which confirmed the effective deposition of polyelectrolytes on both the particles 0.5-µm and 4.5-µm. This was further confirmed with the reversible zeta potential of the particles after deposition of each layer. The SEM images showed the morphology of LbL coated particles covered in thin layers constructed around the polystyrene particles with an uneven surface as compared to plain polystyrene particles having a relatively smooth surface (Figure 1). The in-vitro aerosolization study showed both 0.5-µm and 4.5-µm LbL particles to be inhalable with the mass median aerodynamic diameter (MMAD) of 3.6±0.1 µm and 4.2±0.7 µm with Pari LC nebulizer and 4.2±0.2 µm and 4.5±0.0 µm with AeroEclipse nebulizer, respectively, and more than 70% fine particle fraction (FPF) for both the nebulizers. We were able to passively load 0.07 mg/mg of Dox into the 0.5-µm LbL particles whereas 0.06 mg/mg in 4.5-µm LbL particles. The in-vitro release studies presented a sustained release for both the fabricated particles with ~80% and ~60% drug release from 0.5 and 4.5-µm LbL coated particles in 72 hours (Figure 2). However, the cytotoxicity assessment in Dox resistant H69AR cells demonstrated 0.5-µm LbL particles to be more efficient than 4.5-µm LbL particles with IC50 (0.5-µm) ≤ IC50 (plain dox) ≤ IC50 (4.5-µm) (Figure 3). Therefore, physiologically more relevant in-vitro tumor simulation, 3D-spheroid study was performed for 0.5-µm LbL coated particles and dox at equivalent concentrations of 3.125 and 6.25 µM over a period of 15 days.
Conclusion: The polystyrene particles were successfully coated with successive layers of Chitosan/BSA polyelectrolytes. The deposition was confirmed by increase in the effective diameter and reversible zeta potential of the particles after addition of layers. The SEM images showed the change in surface morphology of the particles on coating with polyelectrolytes. The NGI study showed LbL coated particles are inhalable. Sustained drug release was achieved for the fabricated LbL coated particles. The in-vitro efficacy of the 0.5-µm LbL particles against SCLC cells was achieved which will further be confirmed in an in-vitro tumor simulation study using 3D tumor model mimicking the in-vivo tumorigenic conditions.
Acknowledgements: The authors would like to acknowledge the Imaging Facility of CUNY Advanced Science Research Center for instrument use scientific and technical assistance.
Funding: This project was funded with start-up funds to Vivek Gupta by the College of Pharmacy and Health Sciences, St. John's University, Queens, NY. Gautam Chauhan was supported with the teaching assistantships by St. John's University!
Conflict of Interest: All authors declare no conflict of interest.

Figure 1: SEM images of LbL coated Polystyrene particles before and after deposition of 6 polyelectrolyte layers. The resultant particles preserved a spherical shape and are uniform in size.

Figure 2: In-vitro release profile of Doxorubicin-loaded LbL coated 0.5- and 4.5-µm particles over a period of 3 days. Data represent mean ± SD (n = 3)

Figure 3: Cytotoxicity profile of Doxorubicin, Doxorubicin loaded LbL coated 0.5- and 4.5-µm particles at equivalent Dox concentration in small cell lung cancer (SCLC) cell line H69AR after 72 h of treatment. Data represent the mean ± SD of 2 individual experiments with n = 6 for each experiment.
