Back
Purpose: The aim of this study was to develop formulations for orally administered macromolecules with poor permeability. The formulations need to overcome the poor permeability of the macromolecules across the intestinal membrane and protect them from hydrolysis and enzymatic degradation in the gastrointestinal tract. The absorption of the tested molecules was evaluated using rat or dog animal models.
Methods: A 5 amino acid peptide (molecular weight ~700 g/mol) and an antibody mimetic (molecular weight ~12000 g/mol) were used as model APIs in this study. The APIs were formulated as a suspension, a water-in-oil emulsion or a solution. The formulations contained a lipophilic phase (medium-chain triglyceride [MCT]), lipophilic surfactants (medium-chain diglyceride [MCD]), hydrophilic surfactants (Polysorbate 80 and Kolliphor EL), and solvent (PEG400, triethyl citrate and propylene glycol). To evaluate the effects of lipid digestion on the formulation, 0.5 g of the formulation was added to 50 mL of simulated intestinal fluid and 0.5 mL of pancreatin solution at 250 mg/mL and placed in an in-vitro lipolysis apparatus at 37°C, stirred at 1140 rpm. The peptide formulations were administered to anesthetized Beagle dogs (n=6 animals per group) by intraduodenal instillation using an endoscope. The formulation volume administered to each dog was adjusted to ensure the same weight-based dose in each animal. 1 mL blood samples were collected over several time points, and the API concentration in plasma was determined through bioassay analyses. Pharmacokinetics of the antibody mimetic were studied in Sprague Dawley rats (n=4 animals per group) following direct injection of the formulation into the duodenum of anesthetized animals. Serum samples were collected over several time points and the antibody concentration in serum was quantified using an antibody-specific sandwich ELISA method. Bioavailability was calculated as the ratio of area under the plasma drug concentration-time curve for the test and reference formulation/route of administration. For dog study, an IV administration of the peptide was used as the baseline to calculate oral bioavailability.
Results: The composition of the formulations studied in dogs and their respective oral bioavailability are presented in Table 1. In these formulations, MCT, MCD, and triethyl citrate were used as permeation enhancers, while Tween 80 or Kolliphor EL were used as surfactants to help the digestion kinetic. In addition, water, PEG 400, and propylene glycol were used to solubilize the API in the formulation but had a negative impact on bioavailability. The lipid suspension (Formulation 2) and the water-in-oil emulsion (Formulation 1) had the same level of medium-chain fatty acid released in the in vitro lipolysis experiment. However, the lipid suspension showed a higher bioavailability (37%) than the water-in-oil emulsion (11%). On the other hand, the lipid solution with triethyl citrate as permeation enhancer (formulation 3) only had an oral bioavailability of 12%. Based on these results, only the lipid suspension with a high ratio of MCT formulated without any hydrophilic solvent or co-solvent was tested in the rat models using an antibody mimetic as the API (Table 2). The digestion kinetics of these formulations in the in-vitro lipolysis model was fast: more than 85% of digestible components of the formulation were digested in less than 30 min, releasing medium-chain free fatty acids that are known to increase permeability through the intestinal membrane. Since a suspension formulation was used for the delivery of the antibody mimetic, a higher drug loading can be used as compared to a solution formulation. Therefore, the ratio API/formulation is critical to maximizing the API bioavailability. The bioavailability of the API in the rat model ranged from 2.1 – 2.2% for Formulations 6 and 7 compared to zero absorption for API delivered in PBS only. A high API drug load (Formulation 8) had a negative impact on the API bioavailability (0.4%). This could be attributed to the insufficient quantity of free fatty acid released during digestion to act as permeation enhancers efficiently.
Conclusion: Highly digestible lipid suspension formulations without water (or any other hydrophilic solvent) are beneficial for the bioavailability enhancement of poorly permeable molecules, specifically for BCS III compounds such as proteins and peptides. It is hypothesized that water tends to cause this class of poorly permeable molecules to aggregate. The highest API bioavailability is found in lipid suspension compared to the water-in-oil emulsion. In terms of drug loading, suspension enable to increase API concentration in formulation as the API is dispersed as a powder compared to a solution where the API need to be solubilized in water (or hydrophilic solvent). Finally, the formulation could be encapsulated into a gastro-resistant soft gelatin capsule to further protect the compound from chemical and physical instability in the gastrointestinal tract.
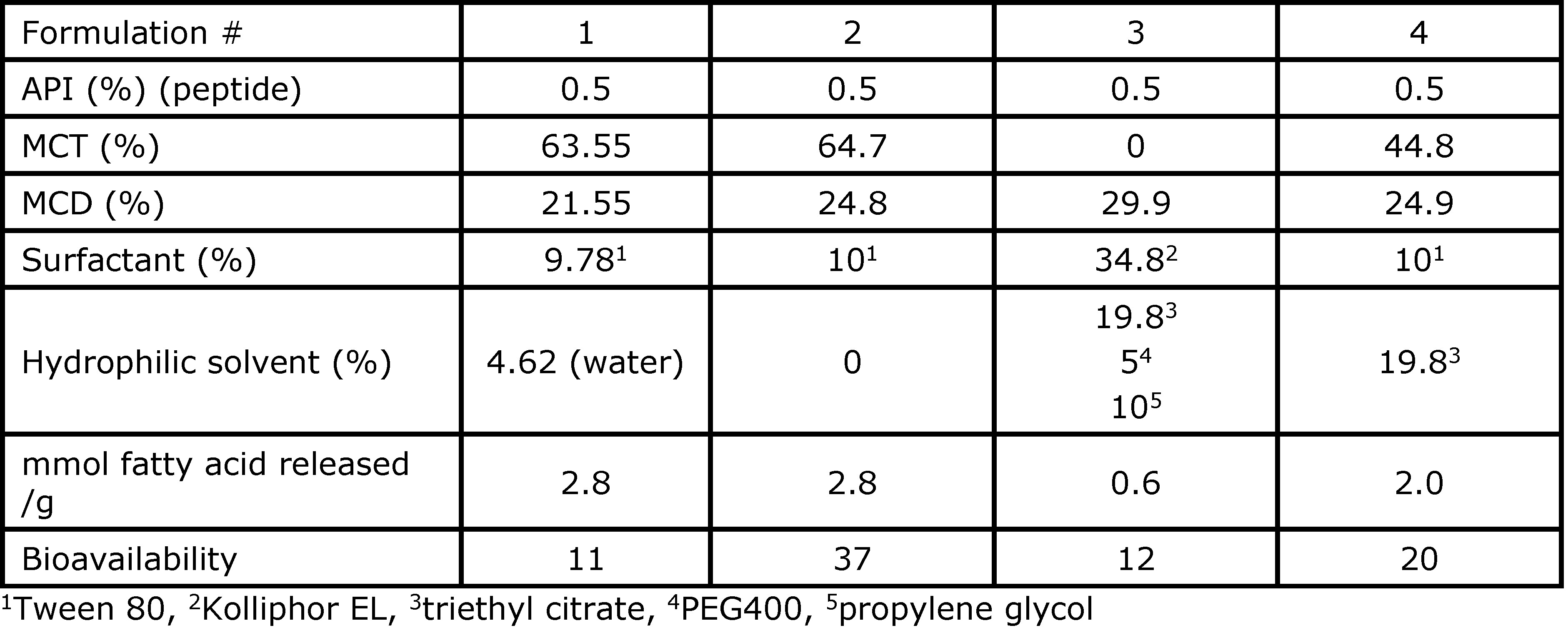
Table 1. Formulations dosed in dogs with the small peptide and their respective oral bioavailability.
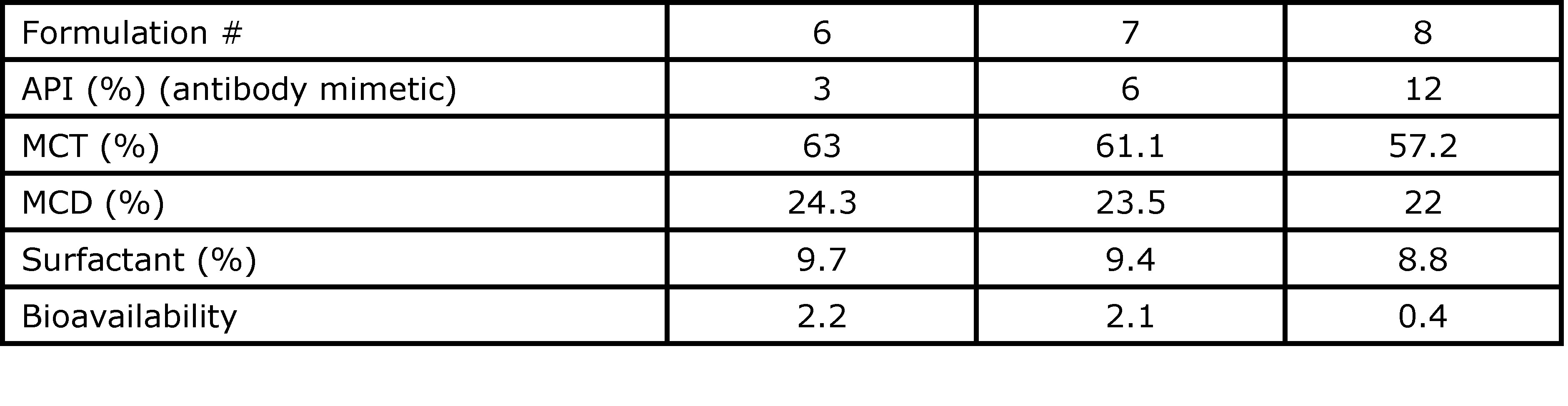
Table 2. Formulations dosed in rats with the antibody mimetic and their respective oral bioavailability
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1030-07-38) Development of Lipid Formulation for Oral Delivery of Proteins and Peptides with Poor Permeability
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- BH
Benoit Hilbold, Ph.D.
Catalent Pharma Solutions
Beinheim, Alsace, France - BH
Benoit Hilbold, Ph.D.
Catalent Pharma Solutions
Beinheim, Alsace, France
Presenting Author(s)
Main Author(s)
Purpose: The aim of this study was to develop formulations for orally administered macromolecules with poor permeability. The formulations need to overcome the poor permeability of the macromolecules across the intestinal membrane and protect them from hydrolysis and enzymatic degradation in the gastrointestinal tract. The absorption of the tested molecules was evaluated using rat or dog animal models.
Methods: A 5 amino acid peptide (molecular weight ~700 g/mol) and an antibody mimetic (molecular weight ~12000 g/mol) were used as model APIs in this study. The APIs were formulated as a suspension, a water-in-oil emulsion or a solution. The formulations contained a lipophilic phase (medium-chain triglyceride [MCT]), lipophilic surfactants (medium-chain diglyceride [MCD]), hydrophilic surfactants (Polysorbate 80 and Kolliphor EL), and solvent (PEG400, triethyl citrate and propylene glycol). To evaluate the effects of lipid digestion on the formulation, 0.5 g of the formulation was added to 50 mL of simulated intestinal fluid and 0.5 mL of pancreatin solution at 250 mg/mL and placed in an in-vitro lipolysis apparatus at 37°C, stirred at 1140 rpm. The peptide formulations were administered to anesthetized Beagle dogs (n=6 animals per group) by intraduodenal instillation using an endoscope. The formulation volume administered to each dog was adjusted to ensure the same weight-based dose in each animal. 1 mL blood samples were collected over several time points, and the API concentration in plasma was determined through bioassay analyses. Pharmacokinetics of the antibody mimetic were studied in Sprague Dawley rats (n=4 animals per group) following direct injection of the formulation into the duodenum of anesthetized animals. Serum samples were collected over several time points and the antibody concentration in serum was quantified using an antibody-specific sandwich ELISA method. Bioavailability was calculated as the ratio of area under the plasma drug concentration-time curve for the test and reference formulation/route of administration. For dog study, an IV administration of the peptide was used as the baseline to calculate oral bioavailability.
Results: The composition of the formulations studied in dogs and their respective oral bioavailability are presented in Table 1. In these formulations, MCT, MCD, and triethyl citrate were used as permeation enhancers, while Tween 80 or Kolliphor EL were used as surfactants to help the digestion kinetic. In addition, water, PEG 400, and propylene glycol were used to solubilize the API in the formulation but had a negative impact on bioavailability. The lipid suspension (Formulation 2) and the water-in-oil emulsion (Formulation 1) had the same level of medium-chain fatty acid released in the in vitro lipolysis experiment. However, the lipid suspension showed a higher bioavailability (37%) than the water-in-oil emulsion (11%). On the other hand, the lipid solution with triethyl citrate as permeation enhancer (formulation 3) only had an oral bioavailability of 12%. Based on these results, only the lipid suspension with a high ratio of MCT formulated without any hydrophilic solvent or co-solvent was tested in the rat models using an antibody mimetic as the API (Table 2). The digestion kinetics of these formulations in the in-vitro lipolysis model was fast: more than 85% of digestible components of the formulation were digested in less than 30 min, releasing medium-chain free fatty acids that are known to increase permeability through the intestinal membrane. Since a suspension formulation was used for the delivery of the antibody mimetic, a higher drug loading can be used as compared to a solution formulation. Therefore, the ratio API/formulation is critical to maximizing the API bioavailability. The bioavailability of the API in the rat model ranged from 2.1 – 2.2% for Formulations 6 and 7 compared to zero absorption for API delivered in PBS only. A high API drug load (Formulation 8) had a negative impact on the API bioavailability (0.4%). This could be attributed to the insufficient quantity of free fatty acid released during digestion to act as permeation enhancers efficiently.
Conclusion: Highly digestible lipid suspension formulations without water (or any other hydrophilic solvent) are beneficial for the bioavailability enhancement of poorly permeable molecules, specifically for BCS III compounds such as proteins and peptides. It is hypothesized that water tends to cause this class of poorly permeable molecules to aggregate. The highest API bioavailability is found in lipid suspension compared to the water-in-oil emulsion. In terms of drug loading, suspension enable to increase API concentration in formulation as the API is dispersed as a powder compared to a solution where the API need to be solubilized in water (or hydrophilic solvent). Finally, the formulation could be encapsulated into a gastro-resistant soft gelatin capsule to further protect the compound from chemical and physical instability in the gastrointestinal tract.

Table 1. Formulations dosed in dogs with the small peptide and their respective oral bioavailability.

Table 2. Formulations dosed in rats with the antibody mimetic and their respective oral bioavailability
