Back
Purpose: The increasing acceptance of amorphous solid dispersions (e.g., twenty-plus approved products since 2010) as a viable formulation technique to enable poorly water-soluble drugs has established spray-drying (SD) and hot-melt extrusion (HME) as leading formulation technologies. Previously, compounds in development exhausted all other "low-risk" solubility-enhancing strategies (i.e., crystal modification, particle size reduction, cyclodextrin complexation, lipid formulations, and pH modification) before exploring amorphization, as this formulation strategy was previously associated with a "high-risk" due to the increased complexity and low chance of success. However, the increasing emergence of poorly-water soluble (i.e., "brick-dust") compounds from development pipelines that are identified to be insoluble in both aqueous and organic solvents, in combination with the adaption of amorphous formulations, has led to the early adoption of amorphous formulation to support pre-clinical studies for new chemical entities (NCEs). In discovery chemistry phases, when progressing toward candidate selection, API amounts can be limited to the milligram or gram scale and carry a heavy cost burden with long lead times for additional quantities. Having limited quantities of API becomes challenging to explore amorphous formulations by hot-melt extrusion due to processing requirements on the gram scale, leaving rotary evaporation and spray-drying as viable solvent methods for assessment. However, spray-drying and rotary evaporation become challenging when the poorly-soluble compounds have limited organic solubility, an emerging trend for the most insoluble compounds. KinetiSol Processing is a next-generation thermokinetic mixing process to generate amorphous solid dispersions that overcome the processing and formulation limitations of HME and SD. Previously, KinetiSol processing required large quantities (e.g., 20 or more grams) of API to assess a compound's compatibility with the technology, limiting it to the same developmental constraints as HME. However, by applying an innovative approach, we hypothesized that we could assess an API's compatibility in our process and various polymers by utilizing low drug loadings (i.e., 2.5%) to conserve API and make an assessment of unknown compounds with limited amounts of API (e.g., 3-5 grams).
Methods: All blends utilized 0.5% Magnesium Stearate and respective polymer with either 2.5% or 20% API. Ten-gram aliquots were loaded into the processing chamber of a lab-scale KinetiSol formulator (Model Number: KF024B). Rotor speeds for two to three stages were programmed, with the ejection triggered by a preset temperature (ranging from 120 – 160C) using an in-chamber infrared probe. A process schematic is shown in figure 1. Previously developed HPLC methods were used for the impurity identification of Abiraterone, Deferasirox, Elacridar, Etravirine, LY3009120, Nintedanib, Omeprazole, Rivaroxaban, Vemurafenib. KinetiSol processed powder samples, and physical mixtures were analyzed for crystalline character by x-ray diffraction (XRD) using a Rigaku MiniFlex 600 benchtop x-ray diffractometer (Rigaku, Inc., Tokyo, Japan). Samples were analyzed in the 2-theta range between 2.5 – 40.0° while rotating. The step size was 0.02°, and the scanning rate was 5.0°/ min.
Results: Nine model drugs were evaluated (i.e., Abiraterone, Deferasirox, Elacridar, Etravirine, LY3009120, Nintedanib, Omeprazole, Rivaroxaban, Vemurafenib) in various polymers (i.e., HPMCAS-LMP, HPMCAS-HMP, MAE 100-55, Copovidone, Soluplus, PVP-K30, and HPMC-E3) at a 2.5% and a 20% drug loading. Identical processing conditions and ejections temperatures were used for each composition at the low and high drug loads to allow correlations to be made about the impact of drug loading on amorphous conversion and impurity formation. 172 KinetiSol experiments were run in total, 86 at the 2.5% drug loading and 86 at the 20% drug loading; figure two plots the impurities of each drug as they correlate to drug loading and the ability of low drug loading to predict the APIs or polymers compatibility accurately. For example, a data point in region II indicates that the low-drug loading approach identified a drug-polymer system to be incompatible at a 2.5% drug loading and, when scaled to 20%, accurately predicted this incompatibility. Similarly, a data point in region IV indicates that the low-drug loading approach identified a compatible drug-polymer system and accurately predicted the compatibility when scaled to 20%. Furthermore, of the 86 KinetiSol experiments ran at the 20% drug load, 7 were crystalline (6 of which were the LY3009120), and all others were amorphous, indicating the predetermined processing conditions selected were aggressive enough to render these "brick-dust" compounds amorphous over 90% of the time. Furthermore, predictive impurity thresholds were created for the 2.5% drug-loaded KinetiSol Experiments in specific polymers at a given temperature, Table 1. The impurity bracketing allows for quick identification of compatible systems with a high probability of success when scaled up.
Conclusion: With the low drug-loading approach, 2 grams of API can be used within KinetiSol processing to evaluate and predict the compatibility of APIs in 8 different polymers (i.e., 250mg API/shot) when API is limited. The low drug-loaded material can be utilized for additional characterization or performance assessment by dissolution evaluation. This innovative approach allows KinetiSol processing to assess the compatibility of new compounds when API is limited.
References: Tan DK, Davis DA, Miller DA, Williams RO, Nokhodchi A. Innovations in thermal processing: hot-melt extrusion and KinetiSol® dispersing. AAPS PharmSciTech. 2020 Nov;21(8):1-20.
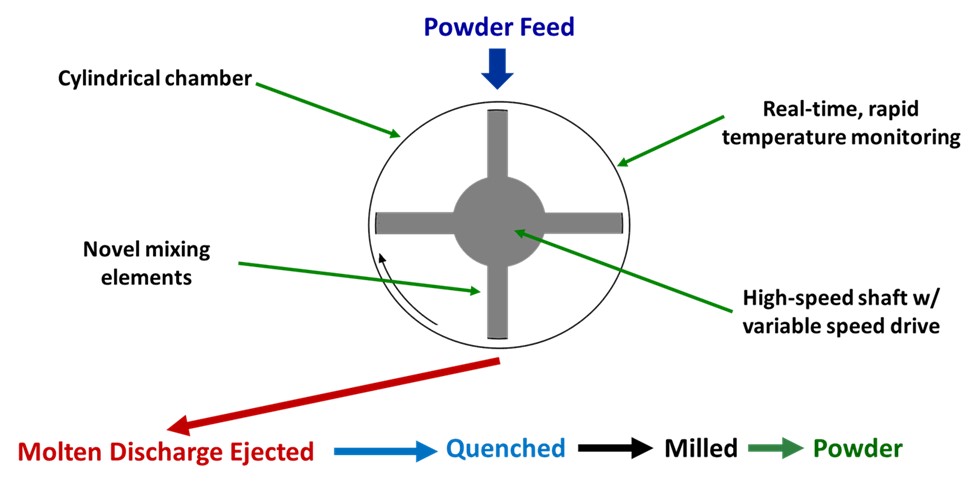
Figure 1: Illustration of KinetiSol Processing to create ASDs
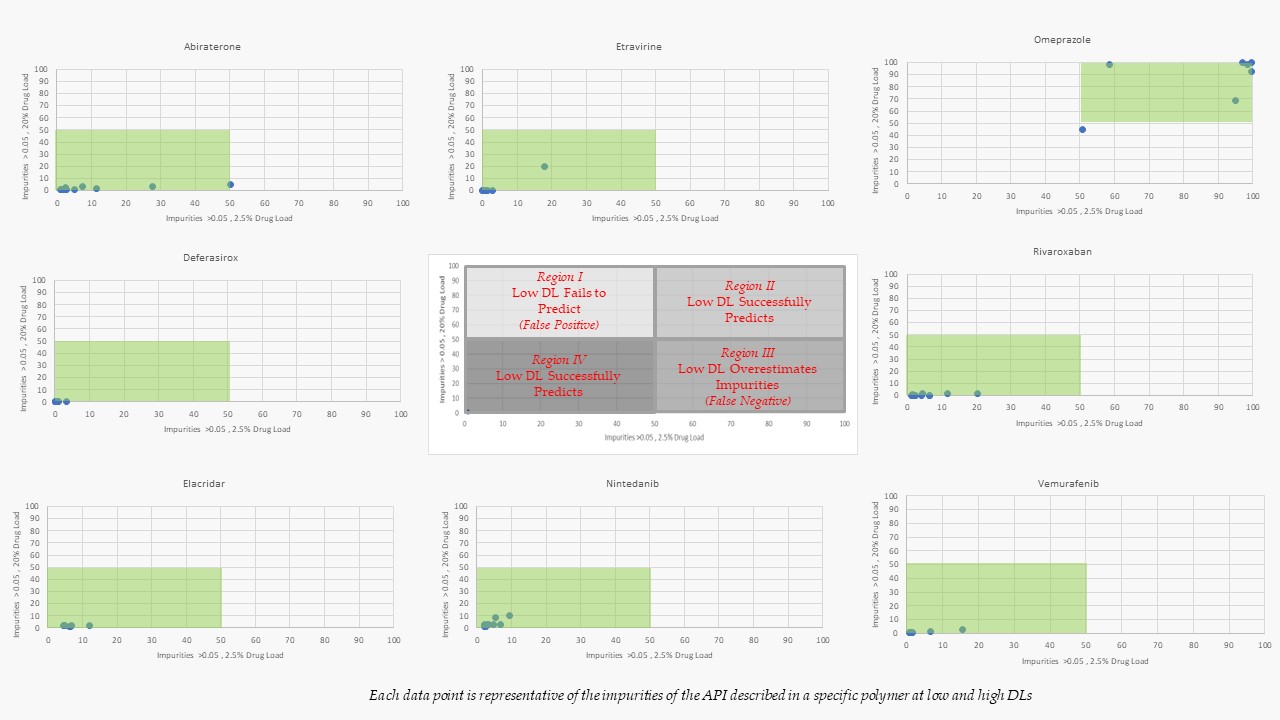
Figure 2: Impurity results for 2.5% and 20% Drug loading in various polymers and API. Green shading highlights the specific region predicted by the screening method.
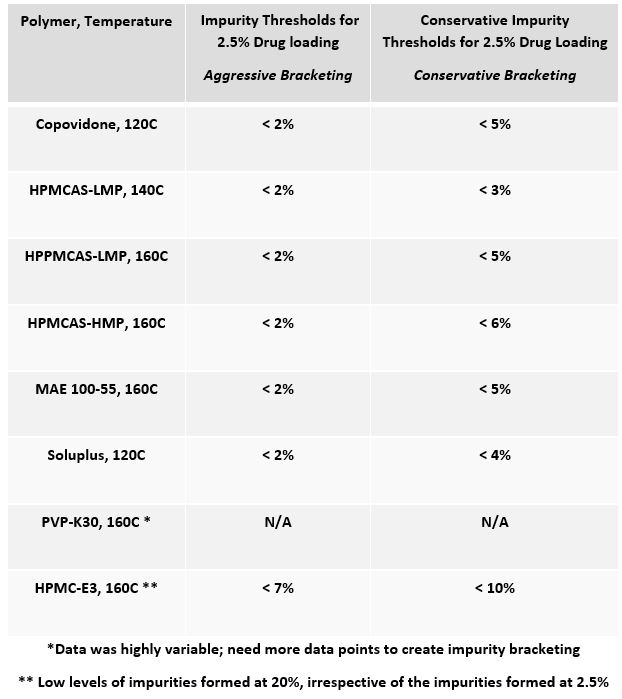
Table 1: Impurity bracketing to predict successful scale-up to a 20% drug loading for a polymer processed at a given temperature, irrespective of API.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(T1330-12-70) API-Sparing KinetiSol Processing Approach to Support Discovery Chemistry
Tuesday, October 18, 2022
1:30 PM – 2:30 PM ET

Daniel A. Davis, Jr., PhD
Scientist
AustinPx
Austin, Texas, United States
Daniel A. Davis, Jr., PhD
Scientist
AustinPx
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: The increasing acceptance of amorphous solid dispersions (e.g., twenty-plus approved products since 2010) as a viable formulation technique to enable poorly water-soluble drugs has established spray-drying (SD) and hot-melt extrusion (HME) as leading formulation technologies. Previously, compounds in development exhausted all other "low-risk" solubility-enhancing strategies (i.e., crystal modification, particle size reduction, cyclodextrin complexation, lipid formulations, and pH modification) before exploring amorphization, as this formulation strategy was previously associated with a "high-risk" due to the increased complexity and low chance of success. However, the increasing emergence of poorly-water soluble (i.e., "brick-dust") compounds from development pipelines that are identified to be insoluble in both aqueous and organic solvents, in combination with the adaption of amorphous formulations, has led to the early adoption of amorphous formulation to support pre-clinical studies for new chemical entities (NCEs). In discovery chemistry phases, when progressing toward candidate selection, API amounts can be limited to the milligram or gram scale and carry a heavy cost burden with long lead times for additional quantities. Having limited quantities of API becomes challenging to explore amorphous formulations by hot-melt extrusion due to processing requirements on the gram scale, leaving rotary evaporation and spray-drying as viable solvent methods for assessment. However, spray-drying and rotary evaporation become challenging when the poorly-soluble compounds have limited organic solubility, an emerging trend for the most insoluble compounds. KinetiSol Processing is a next-generation thermokinetic mixing process to generate amorphous solid dispersions that overcome the processing and formulation limitations of HME and SD. Previously, KinetiSol processing required large quantities (e.g., 20 or more grams) of API to assess a compound's compatibility with the technology, limiting it to the same developmental constraints as HME. However, by applying an innovative approach, we hypothesized that we could assess an API's compatibility in our process and various polymers by utilizing low drug loadings (i.e., 2.5%) to conserve API and make an assessment of unknown compounds with limited amounts of API (e.g., 3-5 grams).
Methods: All blends utilized 0.5% Magnesium Stearate and respective polymer with either 2.5% or 20% API. Ten-gram aliquots were loaded into the processing chamber of a lab-scale KinetiSol formulator (Model Number: KF024B). Rotor speeds for two to three stages were programmed, with the ejection triggered by a preset temperature (ranging from 120 – 160C) using an in-chamber infrared probe. A process schematic is shown in figure 1. Previously developed HPLC methods were used for the impurity identification of Abiraterone, Deferasirox, Elacridar, Etravirine, LY3009120, Nintedanib, Omeprazole, Rivaroxaban, Vemurafenib. KinetiSol processed powder samples, and physical mixtures were analyzed for crystalline character by x-ray diffraction (XRD) using a Rigaku MiniFlex 600 benchtop x-ray diffractometer (Rigaku, Inc., Tokyo, Japan). Samples were analyzed in the 2-theta range between 2.5 – 40.0° while rotating. The step size was 0.02°, and the scanning rate was 5.0°/ min.
Results: Nine model drugs were evaluated (i.e., Abiraterone, Deferasirox, Elacridar, Etravirine, LY3009120, Nintedanib, Omeprazole, Rivaroxaban, Vemurafenib) in various polymers (i.e., HPMCAS-LMP, HPMCAS-HMP, MAE 100-55, Copovidone, Soluplus, PVP-K30, and HPMC-E3) at a 2.5% and a 20% drug loading. Identical processing conditions and ejections temperatures were used for each composition at the low and high drug loads to allow correlations to be made about the impact of drug loading on amorphous conversion and impurity formation. 172 KinetiSol experiments were run in total, 86 at the 2.5% drug loading and 86 at the 20% drug loading; figure two plots the impurities of each drug as they correlate to drug loading and the ability of low drug loading to predict the APIs or polymers compatibility accurately. For example, a data point in region II indicates that the low-drug loading approach identified a drug-polymer system to be incompatible at a 2.5% drug loading and, when scaled to 20%, accurately predicted this incompatibility. Similarly, a data point in region IV indicates that the low-drug loading approach identified a compatible drug-polymer system and accurately predicted the compatibility when scaled to 20%. Furthermore, of the 86 KinetiSol experiments ran at the 20% drug load, 7 were crystalline (6 of which were the LY3009120), and all others were amorphous, indicating the predetermined processing conditions selected were aggressive enough to render these "brick-dust" compounds amorphous over 90% of the time. Furthermore, predictive impurity thresholds were created for the 2.5% drug-loaded KinetiSol Experiments in specific polymers at a given temperature, Table 1. The impurity bracketing allows for quick identification of compatible systems with a high probability of success when scaled up.
Conclusion: With the low drug-loading approach, 2 grams of API can be used within KinetiSol processing to evaluate and predict the compatibility of APIs in 8 different polymers (i.e., 250mg API/shot) when API is limited. The low drug-loaded material can be utilized for additional characterization or performance assessment by dissolution evaluation. This innovative approach allows KinetiSol processing to assess the compatibility of new compounds when API is limited.
References: Tan DK, Davis DA, Miller DA, Williams RO, Nokhodchi A. Innovations in thermal processing: hot-melt extrusion and KinetiSol® dispersing. AAPS PharmSciTech. 2020 Nov;21(8):1-20.

Figure 1: Illustration of KinetiSol Processing to create ASDs

Figure 2: Impurity results for 2.5% and 20% Drug loading in various polymers and API. Green shading highlights the specific region predicted by the screening method.

Table 1: Impurity bracketing to predict successful scale-up to a 20% drug loading for a polymer processed at a given temperature, irrespective of API.
