Back
Purpose: Cystic fibrosis (CF) patients are prone toward the development of recalcitrant bacterial lung infections. A growing concern in the CF community is the isolation of extensively drug resistant (XDR) gram negative pathogens such as Pseudomonas aeruginosa, Burkholderia cepacia complex (1, 2). Failure to treat these chronic infections can result in severe airway inflammation, reduced pulmonary function, respiratory failure and death (3, 5). The utilization of plant-derived, natural products is a promising approach to overcome resistance against known antibiotics, as these products offer an untold diversity of multiple chemical entities which can be enhanced by combination with other drugs or by synthetic chemistry approaches. Curcumin is a natural polyphenolic compound from the rhizome of Curcuma longa (6) which has been previously demonstrated to enhance antibiotic sensitivity against resistant P. aeruginosa isolates through its inhibitory effects on efflux pumps (7-9). Additionally, curcumin has been investigated for its immunomodulatory activity (10), which may be beneficial in attenuating the inflammatory lung injury that typically accompanies opportunistic infections. We hypothesized that addition of curcumin to existing antibiotics could overcome resistance in XDR gram negative pathogens. This hypothesis was tested by examining curcumin activity alone and in combination with colistin sulfate and amikacin in clinical isolates of P. aeruginosa and Burkholderia cepacia complex obtained from CF patients.
Methods: A broth microdilution checkerboard technique (11) was used to determine the potential for additive, synergistic or antagonistic effect of curcumin in combination with colistin sulfate and amikacin against gram negative isolates obtained from CF sputum (provided by Michigan Medicine Division of Pediatric Pulmonology) and standard strain. Stock solutions of curcumin, colistin sulfate, and amikacin were prepared and serially diluted into untreated polystyrene microplates as recommended by CLSI (12). Then, 20 μL of bacterial inoculum (diluted to 5×105 CFU/mL) was added to each well. The plate was incubated at 37°C for 24 hrs under aerobic conditions with shaking. After 24 hrs, antimicrobial activity was assessed using resazurin, an oxidation–reduction indicator. The inhibitory effects of each compound alone and in combination was determined using both visual examination of the resazurin color change from blue to pink as well as quantification by measuring absorbance at 570 nm and normalizing it to untreated growth controls. The ability of the compounds to induce sustained inhibition of bacterial growth was assessed by monitoring resazurin color change over multiple time points (2, 12 and 18 hrs). As a positive control, antimicrobial activity against a colistin and amikacin susceptible P. aeruginosa (ATCC 41501) was monitored.
Results: Curcumin alone exhibited no antimicrobial activity against the P. aeruginosa strains at the concentrations tested (MIC ≥ 4000 µg/mL). In contrast, inhibitory activity of curcumin alone was observed for the clinical isolate A and B of B. cenocepacia (MIC = 500 µg/mL and 125 µg/mL, respectively) and clinical isolate B of B. multivorans tested (MIC = 125 µg/mL) (Table 1). In P. aeruginosa strains that were already susceptible to colistin, the addition of curcumin did not alter the MIC of colistin (i.e., antagonistic activity was not observed). However, in the clinical isolate of P. aeruginosa that exhibited baseline colistin resistance (isolate B, MIC = 64 µg/mL), the addition of curcumin enhanced susceptibility of the bacteria to colistin and a colistin MIC of 2 µg/mL was achieved at a concentration of 250 µg/mL curcumin (Table 1, Fig 1). Enhancement of colistin activity when used in combination with curcumin was also observed for B. cenocepacia isolate B, though this required higher concentrations of each antibiotic compared to that required for P. aeruginosa (Table 1, Fig 1). Overall, curcumin was less effective at overcoming amikacin resistance in Burkholderia cepacia complex isolates. Amikacin activity was enhanced at higher concentrations of curcumin for the B. cenocepacia, but an MIC for amikacin could not be calculated for the B. multivorans strain (Table 1, Fig 2). Of note, while the inhibition of Burkholderia cepacia complex isolates were observed at the first time point (2 hr) of the resazurin assays utilizing curcumin, this inhibition was not sustained over subsequent timepoints (12 and 18 hrs). Further studies will focus on whether degradation of curcumin is the cause of this loss of inhibition, or if other antimicrobial resistance mechanisms are at play. Given the high concentrations of curcumin required for antimicrobial effects, targeted infection site delivery and solubility enhancement are likely to be required for further clinical use.
Conclusion: Curcumin exhibited utility in overcoming antimicrobial resistance to colistin and amikacin in gram negative clinical bacteria isolates. However, the high curcumin doses required for activity present a challenge due to its poor aqueous solubility and chemical instability. Future research efforts will focus on the development of inhalable curcumin formulations that promote compound stability and solubility as well as isolation of the components of the curcuminoid mixture which may be responsible for its antimicrobial activity.>
References: 1. Malhotra S, Hayes D, Jr., Wozniak DJ. Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface. Clin Microbiol Rev. 2019;32(3).
2. Costello A, Reen FJ, O'Gara F, Callaghan M, McClean S. Inhibition of co-colonizing cystic fibrosis-associated pathogens by Pseudomonas aeruginosa and Burkholderia multivorans. Microbiology (Reading). 2014;160(Pt 7):1474-87.
3. Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15(2):194-222.
4. Sousa AM, Pereira MO. Pseudomonas aeruginosa Diversification during Infection Development in Cystic Fibrosis Lungs-A Review. Pathogens. 2014;3(3):680-703.
5. Jones AM, Dodd ME, Govan JR, Barcus V, Doherty CJ, Morris J, et al. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59(11):948-51.
6. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front Pharmacol. 2020;11:01021.
7. Hussain Y, Alam W, Ullah H, Dacrema M, Daglia M, Khan H, et al. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics (Basel). 2022;11(3).
8. Negi N, Prakash P, Gupta ML, Mohapatra TM. Possible Role of Curcumin as an Efflux Pump Inhibitor in Multi Drug Resistant Clinical Isolates of Pseudomonas aeruginosa. J Clin Diagn Res. 2014;8(10):DC04-7.
9. Dai C, Wang Y, Sharma G, Shen J, Velkov T, Xiao X. Polymyxins-Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants (Basel). 2020;9(6).
10. Suresh MV, Wagner MC, Rosania GR, Stringer KA, Min KA, Risler L, et al. Pulmonary administration of a water-soluble curcumin complex reduces severity of acute lung injury. Am J Respir Cell Mol Biol. 2012;47(3):280-7.
11. Berditsch M, Jager T, Strempel N, Schwartz T, Overhage J, Ulrich AS. Synergistic effect of membrane-active peptides polymyxin B and gramicidin S on multidrug-resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59(9):5288-96.
12. CLSI, Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
Acknowledgments: The authors also thank the College of Pharmacy, University of Michigan for support and provide laboratory facilities.
.jpg)
Table 1. Antimicrobial activity of curcumin and antimicrobial drugs against P. aeruginosa, B. cenocepacia and B. multivorans
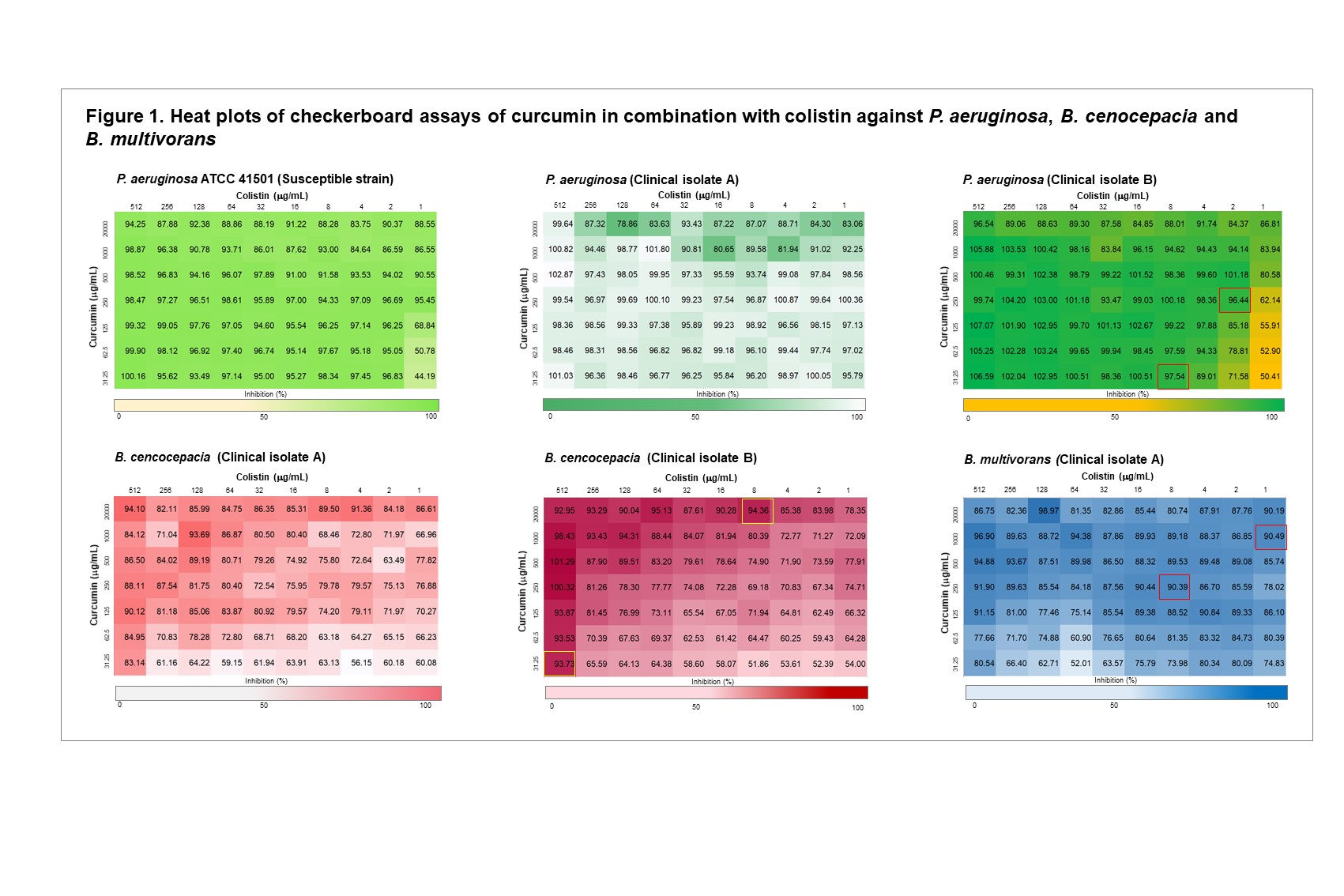
Figure 1. Heat plots of checkerboard assays of curcumin in combination with colistin against P. aeruginosa, B. cenocepacia and B. multivorans
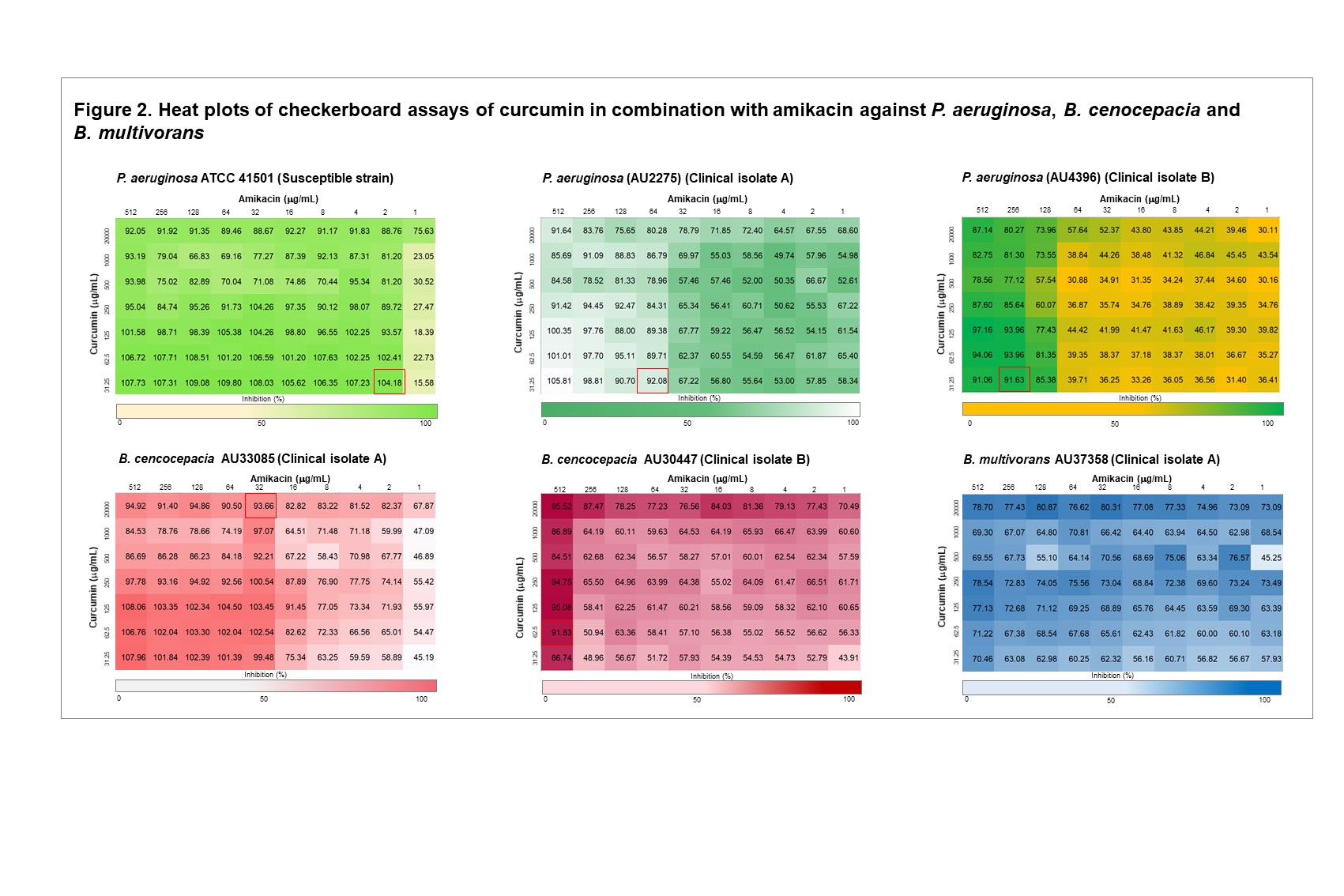
Figure 2. Heat plots of checkerboard assays of curcumin in combination with amikacin against P. aeruginosa, B. cenocepacia and B. multivorans
Discovery and Basic Research - Pharmacology
Category: Late Breaking Poster Abstract
(T1230-05-29) Curcumin as a Repurposed Adjuvant to Overcome Antibiotic Resistance in Pseudomonas aeruginosa, Burkholderia cenocepacia and Burkholderia multivorans Clinical Isolates
Tuesday, October 18, 2022
12:30 PM – 1:30 PM ET
- BD
Bhanuz Dechayont, MS
University of Michigan
Ann Arbor, Michigan, United States - BD
Bhanuz Dechayont, MS
University of Michigan
Ann Arbor, Michigan, United States
Presenting Author(s)
Main Author(s)
Purpose: Cystic fibrosis (CF) patients are prone toward the development of recalcitrant bacterial lung infections. A growing concern in the CF community is the isolation of extensively drug resistant (XDR) gram negative pathogens such as Pseudomonas aeruginosa, Burkholderia cepacia complex (1, 2). Failure to treat these chronic infections can result in severe airway inflammation, reduced pulmonary function, respiratory failure and death (3, 5). The utilization of plant-derived, natural products is a promising approach to overcome resistance against known antibiotics, as these products offer an untold diversity of multiple chemical entities which can be enhanced by combination with other drugs or by synthetic chemistry approaches. Curcumin is a natural polyphenolic compound from the rhizome of Curcuma longa (6) which has been previously demonstrated to enhance antibiotic sensitivity against resistant P. aeruginosa isolates through its inhibitory effects on efflux pumps (7-9). Additionally, curcumin has been investigated for its immunomodulatory activity (10), which may be beneficial in attenuating the inflammatory lung injury that typically accompanies opportunistic infections. We hypothesized that addition of curcumin to existing antibiotics could overcome resistance in XDR gram negative pathogens. This hypothesis was tested by examining curcumin activity alone and in combination with colistin sulfate and amikacin in clinical isolates of P. aeruginosa and Burkholderia cepacia complex obtained from CF patients.
Methods: A broth microdilution checkerboard technique (11) was used to determine the potential for additive, synergistic or antagonistic effect of curcumin in combination with colistin sulfate and amikacin against gram negative isolates obtained from CF sputum (provided by Michigan Medicine Division of Pediatric Pulmonology) and standard strain. Stock solutions of curcumin, colistin sulfate, and amikacin were prepared and serially diluted into untreated polystyrene microplates as recommended by CLSI (12). Then, 20 μL of bacterial inoculum (diluted to 5×105 CFU/mL) was added to each well. The plate was incubated at 37°C for 24 hrs under aerobic conditions with shaking. After 24 hrs, antimicrobial activity was assessed using resazurin, an oxidation–reduction indicator. The inhibitory effects of each compound alone and in combination was determined using both visual examination of the resazurin color change from blue to pink as well as quantification by measuring absorbance at 570 nm and normalizing it to untreated growth controls. The ability of the compounds to induce sustained inhibition of bacterial growth was assessed by monitoring resazurin color change over multiple time points (2, 12 and 18 hrs). As a positive control, antimicrobial activity against a colistin and amikacin susceptible P. aeruginosa (ATCC 41501) was monitored.
Results: Curcumin alone exhibited no antimicrobial activity against the P. aeruginosa strains at the concentrations tested (MIC ≥ 4000 µg/mL). In contrast, inhibitory activity of curcumin alone was observed for the clinical isolate A and B of B. cenocepacia (MIC = 500 µg/mL and 125 µg/mL, respectively) and clinical isolate B of B. multivorans tested (MIC = 125 µg/mL) (Table 1). In P. aeruginosa strains that were already susceptible to colistin, the addition of curcumin did not alter the MIC of colistin (i.e., antagonistic activity was not observed). However, in the clinical isolate of P. aeruginosa that exhibited baseline colistin resistance (isolate B, MIC = 64 µg/mL), the addition of curcumin enhanced susceptibility of the bacteria to colistin and a colistin MIC of 2 µg/mL was achieved at a concentration of 250 µg/mL curcumin (Table 1, Fig 1). Enhancement of colistin activity when used in combination with curcumin was also observed for B. cenocepacia isolate B, though this required higher concentrations of each antibiotic compared to that required for P. aeruginosa (Table 1, Fig 1). Overall, curcumin was less effective at overcoming amikacin resistance in Burkholderia cepacia complex isolates. Amikacin activity was enhanced at higher concentrations of curcumin for the B. cenocepacia, but an MIC for amikacin could not be calculated for the B. multivorans strain (Table 1, Fig 2). Of note, while the inhibition of Burkholderia cepacia complex isolates were observed at the first time point (2 hr) of the resazurin assays utilizing curcumin, this inhibition was not sustained over subsequent timepoints (12 and 18 hrs). Further studies will focus on whether degradation of curcumin is the cause of this loss of inhibition, or if other antimicrobial resistance mechanisms are at play. Given the high concentrations of curcumin required for antimicrobial effects, targeted infection site delivery and solubility enhancement are likely to be required for further clinical use.
Conclusion: Curcumin exhibited utility in overcoming antimicrobial resistance to colistin and amikacin in gram negative clinical bacteria isolates. However, the high curcumin doses required for activity present a challenge due to its poor aqueous solubility and chemical instability. Future research efforts will focus on the development of inhalable curcumin formulations that promote compound stability and solubility as well as isolation of the components of the curcuminoid mixture which may be responsible for its antimicrobial activity.>
References: 1. Malhotra S, Hayes D, Jr., Wozniak DJ. Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface. Clin Microbiol Rev. 2019;32(3).
2. Costello A, Reen FJ, O'Gara F, Callaghan M, McClean S. Inhibition of co-colonizing cystic fibrosis-associated pathogens by Pseudomonas aeruginosa and Burkholderia multivorans. Microbiology (Reading). 2014;160(Pt 7):1474-87.
3. Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15(2):194-222.
4. Sousa AM, Pereira MO. Pseudomonas aeruginosa Diversification during Infection Development in Cystic Fibrosis Lungs-A Review. Pathogens. 2014;3(3):680-703.
5. Jones AM, Dodd ME, Govan JR, Barcus V, Doherty CJ, Morris J, et al. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax. 2004;59(11):948-51.
6. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front Pharmacol. 2020;11:01021.
7. Hussain Y, Alam W, Ullah H, Dacrema M, Daglia M, Khan H, et al. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics (Basel). 2022;11(3).
8. Negi N, Prakash P, Gupta ML, Mohapatra TM. Possible Role of Curcumin as an Efflux Pump Inhibitor in Multi Drug Resistant Clinical Isolates of Pseudomonas aeruginosa. J Clin Diagn Res. 2014;8(10):DC04-7.
9. Dai C, Wang Y, Sharma G, Shen J, Velkov T, Xiao X. Polymyxins-Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants (Basel). 2020;9(6).
10. Suresh MV, Wagner MC, Rosania GR, Stringer KA, Min KA, Risler L, et al. Pulmonary administration of a water-soluble curcumin complex reduces severity of acute lung injury. Am J Respir Cell Mol Biol. 2012;47(3):280-7.
11. Berditsch M, Jager T, Strempel N, Schwartz T, Overhage J, Ulrich AS. Synergistic effect of membrane-active peptides polymyxin B and gramicidin S on multidrug-resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59(9):5288-96.
12. CLSI, Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
Acknowledgments: The authors also thank the College of Pharmacy, University of Michigan for support and provide laboratory facilities.
.jpg)
Table 1. Antimicrobial activity of curcumin and antimicrobial drugs against P. aeruginosa, B. cenocepacia and B. multivorans

Figure 1. Heat plots of checkerboard assays of curcumin in combination with colistin against P. aeruginosa, B. cenocepacia and B. multivorans

Figure 2. Heat plots of checkerboard assays of curcumin in combination with amikacin against P. aeruginosa, B. cenocepacia and B. multivorans
