Back
Purpose: Long-acting injectable drug formulations are desired to reduce side effect and dose frequency, and subsequently promote patient adherence. However, time-consuming manufacturing process, limited dosing, initial burst, injection site reactions and thicker injection needle are obstacles towards a broad usage. In our group, cholesterol-modified hyaluronic acid (CHHA), forming nano-sized hydrogel (HA nanogel) in water, having versatile encapsulation property of small-sized molecules, middle-sized molecules, peptides including cyclic peptides, and proteins, have been developed. The HA nanogels also showed unique colloidal property in which nanogel-clustering precipitates are formed in response to high ionic strength in the body. Nanogel-clustering enabled in situ gelling and demonstrated sustained release of rhGH in rats over the course of 1 week. Herein, dual-drug delivery system, consisting of dissolution-controlled delivery system based on water-insoluble nanocrystals of cyclic peptides and in situ gelling delivery system of HA nanogel, was designed for long-acting formulation and studied in vivo.
Methods: The cyclosporine A (CyA) was dispersed in an aqueous solution containing 5 mg/mL Polysorbate 80 and milled using zirconium oxide beads with a diameter of 0.2 mm in a 30 mL glass vial for 11 days. The suspension was washed with water for injection twice using centrifugation. The particle size and concentration of the nanocrystal were analyzed with DLS and RP-HPLC. The in situ gelling suspensions were prepared by dispersing the desired amounts of nanocrystal into the HA nanogel (Mw of HA: 35k, Degree of substitution of cholesterol: 19%) with 9% sucrose. The in situ gelling suspensions or suspension formulation without HA nanogel were injected subcutaneously into the six-week-old male Sprague-Dawley rats with a 25-gauge needle at a dose of 25 mg/kg as CyA. The solution formulation of CyA with 10%αCD was also injected subcutaneously (0.5mg/kg) for the control. Blood was collected from the jugular vein and CyA levels in plasma were determined using a LC-MS/MS method. The pharmacokinetic parameter was assessed with non-compartmental analysis, using WinNonlin (version 8.3).
Results: A nanocrystal of CyA with a diameter of 171 nm was successfully prepared after beads milling. The in situ gelling nanosuspensions of 10 mg/mL CyA were formulated by just dispersing the nanocrystal into the HA nanogel solution (5 mg/mL or 10 mg/mL). These formulations were milk-like colloidal suspension and can go through a 30G needle. The CyA plasma concentration profile in mice was assessed for the in situ gelling nanosuspension formulation with HA nanogel and the nanosuspension formulation without HA nanogel. The pharmacokinetic (PK) profiles of CyA are shown in Figure 1. The in situ gelling nanosuspension formulations maintained plasma CyA levels within a narrow range over 1 month without initial burst compared with the nanosuspension formulation without HA nanogel. The mean residence time (MRT) of in situ gelling formulations was 690 h (10 mg/mL HA nanogel) and 518 h (5mg/mL HA nanogel), which were much longer than that of nanosuspension formulation without HA nanogel at 183 h. The results showed that HA nanogel efficiently helped controlled release of CyA.
Conclusion: From the above results, we have concluded that the in situ gelling nanosuspension formulation with HA nanogel have enormous potential to enhance the duration of a drug in the body for a long period reducing the initial burst. We believe this dual-drug delivery system with HA nanogel can realize the long-acting injectable drug products and show a great impact on the patient’s life and their family’s life.
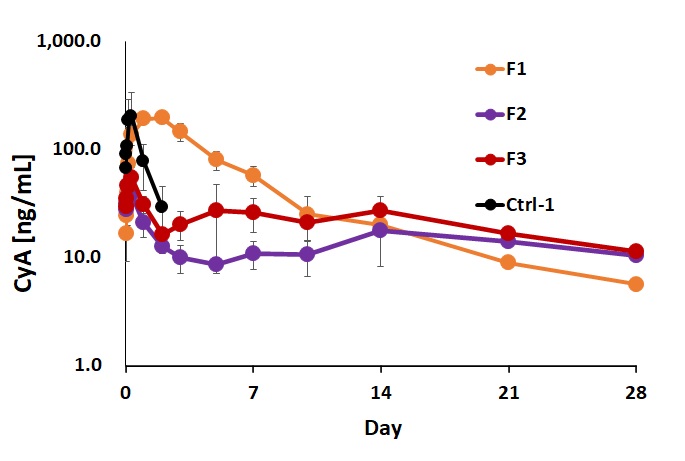
F1: The nanosuspension formulation of 10mg/mL CyA without HA nanogel.
F2: The in situ gelling nanosuspension formulation of 10mg/mL CyA with 10mg/mL HA nanogel.
F3: The in situ gelling nanosuspension formulation of 10mg/mL CyA with 5mg/mL HA nanogel.
Ctrl-1:The solution formulation of 0.5mg/mL CyA with 10%αCD.
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1430-04-19) Facile Dual-Drug Delivery System Based on In Situ Gelling Nanosuspension Formulation of Water-Insoluble Cyclic Peptides
Monday, October 17, 2022
2:30 PM – 3:30 PM ET
- TN
Takashi Nakai, Ph.D.
Asahi Kasei Corporation
FUJI-SHI, Shizuoka, Japan - TN
Takashi Nakai, Ph.D.
Asahi Kasei Corporation
FUJI-SHI, Shizuoka, Japan
Presenting Author(s)
Main Author(s)
Purpose: Long-acting injectable drug formulations are desired to reduce side effect and dose frequency, and subsequently promote patient adherence. However, time-consuming manufacturing process, limited dosing, initial burst, injection site reactions and thicker injection needle are obstacles towards a broad usage. In our group, cholesterol-modified hyaluronic acid (CHHA), forming nano-sized hydrogel (HA nanogel) in water, having versatile encapsulation property of small-sized molecules, middle-sized molecules, peptides including cyclic peptides, and proteins, have been developed. The HA nanogels also showed unique colloidal property in which nanogel-clustering precipitates are formed in response to high ionic strength in the body. Nanogel-clustering enabled in situ gelling and demonstrated sustained release of rhGH in rats over the course of 1 week. Herein, dual-drug delivery system, consisting of dissolution-controlled delivery system based on water-insoluble nanocrystals of cyclic peptides and in situ gelling delivery system of HA nanogel, was designed for long-acting formulation and studied in vivo.
Methods: The cyclosporine A (CyA) was dispersed in an aqueous solution containing 5 mg/mL Polysorbate 80 and milled using zirconium oxide beads with a diameter of 0.2 mm in a 30 mL glass vial for 11 days. The suspension was washed with water for injection twice using centrifugation. The particle size and concentration of the nanocrystal were analyzed with DLS and RP-HPLC. The in situ gelling suspensions were prepared by dispersing the desired amounts of nanocrystal into the HA nanogel (Mw of HA: 35k, Degree of substitution of cholesterol: 19%) with 9% sucrose. The in situ gelling suspensions or suspension formulation without HA nanogel were injected subcutaneously into the six-week-old male Sprague-Dawley rats with a 25-gauge needle at a dose of 25 mg/kg as CyA. The solution formulation of CyA with 10%αCD was also injected subcutaneously (0.5mg/kg) for the control. Blood was collected from the jugular vein and CyA levels in plasma were determined using a LC-MS/MS method. The pharmacokinetic parameter was assessed with non-compartmental analysis, using WinNonlin (version 8.3).
Results: A nanocrystal of CyA with a diameter of 171 nm was successfully prepared after beads milling. The in situ gelling nanosuspensions of 10 mg/mL CyA were formulated by just dispersing the nanocrystal into the HA nanogel solution (5 mg/mL or 10 mg/mL). These formulations were milk-like colloidal suspension and can go through a 30G needle. The CyA plasma concentration profile in mice was assessed for the in situ gelling nanosuspension formulation with HA nanogel and the nanosuspension formulation without HA nanogel. The pharmacokinetic (PK) profiles of CyA are shown in Figure 1. The in situ gelling nanosuspension formulations maintained plasma CyA levels within a narrow range over 1 month without initial burst compared with the nanosuspension formulation without HA nanogel. The mean residence time (MRT) of in situ gelling formulations was 690 h (10 mg/mL HA nanogel) and 518 h (5mg/mL HA nanogel), which were much longer than that of nanosuspension formulation without HA nanogel at 183 h. The results showed that HA nanogel efficiently helped controlled release of CyA.
Conclusion: From the above results, we have concluded that the in situ gelling nanosuspension formulation with HA nanogel have enormous potential to enhance the duration of a drug in the body for a long period reducing the initial burst. We believe this dual-drug delivery system with HA nanogel can realize the long-acting injectable drug products and show a great impact on the patient’s life and their family’s life.

F1: The nanosuspension formulation of 10mg/mL CyA without HA nanogel.
F2: The in situ gelling nanosuspension formulation of 10mg/mL CyA with 10mg/mL HA nanogel.
F3: The in situ gelling nanosuspension formulation of 10mg/mL CyA with 5mg/mL HA nanogel.
Ctrl-1:The solution formulation of 0.5mg/mL CyA with 10%αCD.
