Back
Purpose: Tuberculosis is a granulomatous infection caused by the bacterial pathogen Mycobacterium tuberculosis (M. tb) and is one of the major causes of death globally. Owing to increasing drug resistance and poor activity of existing therapies, there is a clear need for novel therapies that can address these limitations. Therefore, a novel urea-based compound (1235) was discovered achieving a potent minimum inhibitory concentration (MIC) value of 0.01 μg/mL against M. tb (H37Rv)1. However, the major limitation of this novel compound as observed from various pre-formulation studies is its low aqueous solubility and high lipophilicity. Therefore, this study focuses on increasing the solubility of this novel compound to enhance aqueous solubility through cyclodextrin inclusion complex formation as the drug can effectively bind to the hydrophobic cavity.
Methods: The in-silico platforms SwissADME and pKCSM, which provide free access to a variety of quick yet accurate forecasting models for physicochemical parameters, were employed to determine theoretical physicochemical/pharmacokinetic properties. Experimental kinetic solubility of 1235 was determined. To screen a suitable cyclodextrin effectively and rapidly for inclusion complex, an in-silico strategy was utilized using AutoDock software to dock the novel antitubercular compound inside the hydrophobic cavity of each commercially available cyclodextrin (αCD, βCD, γCD, HP-β-CD, SBE-β-CD). The binding affinity of the drug cyclodextrin complex derived from docking was considered for selection of cyclodextrin. Thermodynamic solubility study was performed with selected cyclodextrin to determine optimal stoichiometric ratio of drug and cyclodextrin to form inclusion complex2. With the obtained stoichiometric ratio from phase solubility study cyclodextrin inclusion complex was prepared using, co-precipitation and solvent evaporation method. Further the inclusion complex formation was confirmed by characterizations such as differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR).
Results: The preliminary studies performed using in silico platforms like SwissADME and pKCSM revealed that the cLogP of the compound 1235 was 3.94 and 4.05, respectively, suggesting appropriate physicochemical properties for oral bioavailability. Furthermore, the theoretical solubility of the compound was determined to be 2.86 µg/mL and 1.99 µg/mL, respectively. Experimentally, and the kinetic solubility of 1235 was obtained to be < 0.1 µg/mL, which is lower than the predicted values. The docking study conducted on various cyclodextrins with 1235 reveals that the binding affinity of the compound was highest for HP-β-CD followed by SBE-β-CD, βCD, γCD and the binding affinity was obtained as -7.4 kcal/mol, -6.6 kcal/mol, -6.4 kcal/mol and -6.2 kcal/mol, respectively. αCD was not able to form a stable complex with the novel compound. This could possibly be due to a smaller cavity size. Thermodynamic solubility was conducted using HP-β-CD revealed that the stoichiometric ratio of 1:1 of drug: cyclodextrin forms a stable inclusion complex. In addition, formation of the inclusion complex was further supported using DSC, FTIR, and NMR
Conclusion: A novel urea-based compound (1235) was formulated with HP-β-CD using the co-precipitation method and solvent evaporation method, was then also characterized by DSC, FTIR, and NMR, it was confirmed that the novel compound was successfully able to form an inclusion complex, and eventually increases its solubility. As a result, a suitable drug delivery system is needed to test the efficacy of this formulation in vivo.
References: (1) Brown, J. R.; North, E. J.; Hurdle, J. G.; Morisseau, C.; Scarborough, J. S.; Sun, D.; Korduláková, J.; Scherman, M. S.; Jones, V.; Grzegorzewicz, A.; Crew, R. M.; Jackson, M.; McNeil, M. R.; Lee, R. E. The Structure-Activity Relationship of Urea Derivatives as Anti-Tuberculosis Agents. Bioorg. Med. Chem. 2011, 19 (18), 5585–5595. https://doi.org/10.1016/j.bmc.2011.07.034.
(2) Wang, L.; Li, S.; Tang, P.; Yan, J.; Xu, K.; Li, H. Characterization and Evaluation of Synthetic Riluzole with β-Cyclodextrin and 2,6-Di-O-Methyl-β-Cyclodextrin Inclusion Complexes. Carbohydr. Polym. 2015, 129, 9–16. https://doi.org/10.1016/j.carbpol.2015.04.046.
Acknowledgments: The authors are grateful for Creighton University Department of Chemistry and Biochemistry for the use of the 400 MHz Bruker NMR.
Funding: The authors gratefully thank the National Institutes of Health (R15 AI164443 and P20 GM139762-01) for funding this project.
Conflict of Interest Statements: We confirm that there are no conflicts of interest associated with this work and there has been no significant financial support for this work that could have influenced its outcome.
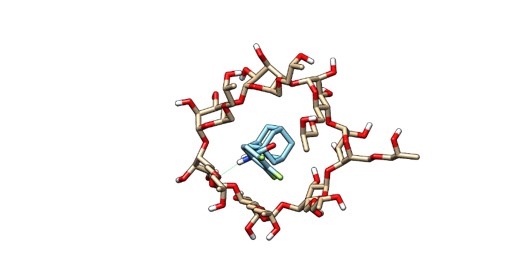
Fig 1. Docking of novel compound (1235) with HP-β-CD
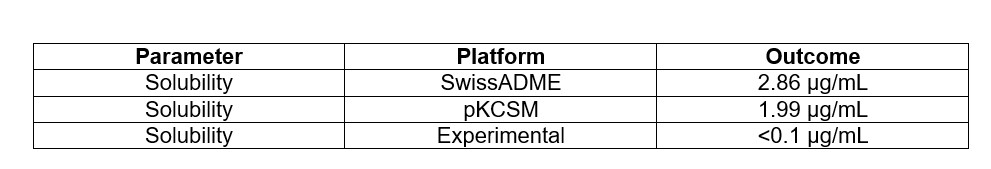
Table 1. Solubility in-silico and Experimental
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1230-12-70) Cyclodextrin Inclusion Complex of Novel Anti-mycobacterial Compound
Monday, October 17, 2022
12:30 PM – 1:30 PM ET
- AS
Amey Sukhia, BS
Creighton University
Omaha, Nebraska, United States - AS
Amey Sukhia, BS
Creighton University
Omaha, Nebraska, United States
Presenting Author(s)
Main Author(s)
Purpose: Tuberculosis is a granulomatous infection caused by the bacterial pathogen Mycobacterium tuberculosis (M. tb) and is one of the major causes of death globally. Owing to increasing drug resistance and poor activity of existing therapies, there is a clear need for novel therapies that can address these limitations. Therefore, a novel urea-based compound (1235) was discovered achieving a potent minimum inhibitory concentration (MIC) value of 0.01 μg/mL against M. tb (H37Rv)1. However, the major limitation of this novel compound as observed from various pre-formulation studies is its low aqueous solubility and high lipophilicity. Therefore, this study focuses on increasing the solubility of this novel compound to enhance aqueous solubility through cyclodextrin inclusion complex formation as the drug can effectively bind to the hydrophobic cavity.
Methods: The in-silico platforms SwissADME and pKCSM, which provide free access to a variety of quick yet accurate forecasting models for physicochemical parameters, were employed to determine theoretical physicochemical/pharmacokinetic properties. Experimental kinetic solubility of 1235 was determined. To screen a suitable cyclodextrin effectively and rapidly for inclusion complex, an in-silico strategy was utilized using AutoDock software to dock the novel antitubercular compound inside the hydrophobic cavity of each commercially available cyclodextrin (αCD, βCD, γCD, HP-β-CD, SBE-β-CD). The binding affinity of the drug cyclodextrin complex derived from docking was considered for selection of cyclodextrin. Thermodynamic solubility study was performed with selected cyclodextrin to determine optimal stoichiometric ratio of drug and cyclodextrin to form inclusion complex2. With the obtained stoichiometric ratio from phase solubility study cyclodextrin inclusion complex was prepared using, co-precipitation and solvent evaporation method. Further the inclusion complex formation was confirmed by characterizations such as differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR).
Results: The preliminary studies performed using in silico platforms like SwissADME and pKCSM revealed that the cLogP of the compound 1235 was 3.94 and 4.05, respectively, suggesting appropriate physicochemical properties for oral bioavailability. Furthermore, the theoretical solubility of the compound was determined to be 2.86 µg/mL and 1.99 µg/mL, respectively. Experimentally, and the kinetic solubility of 1235 was obtained to be < 0.1 µg/mL, which is lower than the predicted values. The docking study conducted on various cyclodextrins with 1235 reveals that the binding affinity of the compound was highest for HP-β-CD followed by SBE-β-CD, βCD, γCD and the binding affinity was obtained as -7.4 kcal/mol, -6.6 kcal/mol, -6.4 kcal/mol and -6.2 kcal/mol, respectively. αCD was not able to form a stable complex with the novel compound. This could possibly be due to a smaller cavity size. Thermodynamic solubility was conducted using HP-β-CD revealed that the stoichiometric ratio of 1:1 of drug: cyclodextrin forms a stable inclusion complex. In addition, formation of the inclusion complex was further supported using DSC, FTIR, and NMR
Conclusion: A novel urea-based compound (1235) was formulated with HP-β-CD using the co-precipitation method and solvent evaporation method, was then also characterized by DSC, FTIR, and NMR, it was confirmed that the novel compound was successfully able to form an inclusion complex, and eventually increases its solubility. As a result, a suitable drug delivery system is needed to test the efficacy of this formulation in vivo.
References: (1) Brown, J. R.; North, E. J.; Hurdle, J. G.; Morisseau, C.; Scarborough, J. S.; Sun, D.; Korduláková, J.; Scherman, M. S.; Jones, V.; Grzegorzewicz, A.; Crew, R. M.; Jackson, M.; McNeil, M. R.; Lee, R. E. The Structure-Activity Relationship of Urea Derivatives as Anti-Tuberculosis Agents. Bioorg. Med. Chem. 2011, 19 (18), 5585–5595. https://doi.org/10.1016/j.bmc.2011.07.034.
(2) Wang, L.; Li, S.; Tang, P.; Yan, J.; Xu, K.; Li, H. Characterization and Evaluation of Synthetic Riluzole with β-Cyclodextrin and 2,6-Di-O-Methyl-β-Cyclodextrin Inclusion Complexes. Carbohydr. Polym. 2015, 129, 9–16. https://doi.org/10.1016/j.carbpol.2015.04.046.
Acknowledgments: The authors are grateful for Creighton University Department of Chemistry and Biochemistry for the use of the 400 MHz Bruker NMR.
Funding: The authors gratefully thank the National Institutes of Health (R15 AI164443 and P20 GM139762-01) for funding this project.
Conflict of Interest Statements: We confirm that there are no conflicts of interest associated with this work and there has been no significant financial support for this work that could have influenced its outcome.

Fig 1. Docking of novel compound (1235) with HP-β-CD

Table 1. Solubility in-silico and Experimental
