Back
Purpose: Cancer immunotherapy has rapidly emerged and advanced in the past few years. The success of immunotherapy relies on delicately harnessing the power of the immune system, including modulating immune functions using cytokines, inhibiting immune checkpoints, and manipulating immune cells to recognize cancer cells (1). The immune system comprises a variety of tissues, cell types, and molecules, which are intricately connected and highly regulated. Therefore, therapeutics modulating the immune system could sometimes become double-edged swords and result in collateral damage on normal tissues. Severe and even fatal adverse effects have been reported during clinical trials of immunomodulatory therapeutics highlighting the need for assessing immunotoxicity in preclinical studies (2, 3). Currently, regulatory guidance from both Food and Drug Administration and European Medicines Agency recommend a weight-of-evidence approach to assess the immunotoxicity potential of drugs and biologics (4, 5). One of the preclinical immunotoxicity study recommended is immunophenotyping in order to identify the specific cell populations affected and to provide useful clinical biomarkers (5). Given the physiological relevance, non-human primates, especially cynomolgus monkeys, are widely used for immunotoxicological testing of pharmaceutical reagents. The objective of this report is to establish a reliable lymphocyte immunophenotyping reference range in cynomolgus monkey, which could significantly aid in the immunotoxicological assessment in preclinical study.
Methods: Animals - Cynomolgus monkeys used in this report were originated from China. All animals were naïve and maintained in compliance with the Guide for the Care and Use of Laboratory Animals. All animal research was approved by the Institutional Animal Care and Use Committee. Immunophenotyping - Blood samples were collected with anti-coagulant potassium EDTA and washed with 2 mL of DPBS by centrifugation of 5 min at 400 g. Antibody cocktail was added which includes: FITC anti-NHP CD45, V450 anti-human CD3, PerCP-Cy5.5 anti-human CD4, PE anti-human CD8, APC anti-human CD20, and BV 510 anti-human CD16. Cells were incubated with antibody cocktail at room temperature for 20 to 30 minutes protected from white light. Cells were then washed and re-suspended with DPBS and analyzed using BD LSRFortessaTM flow cytometer with FACS Diva software.
Results: Lymphocyte immunophenotyping was conducted in peripheral blood collected from 560 untreated male and 578 untreated female cynomolgus monkeys. The percentage of lymphocyte subpopulations was reported in Table 1, which includes total T cell (CD3+), helper T cell (CD3+ CD4+ CD8-), cytotoxic T cell (CD3+ CD4- CD8+), B cell (CD3- CD20+), and natural killer (NK) cell (CD3- CD16+). To assess the distribution of the data, a variety of parameters were calculated including the mean, standard deviation, minimum, 2.5% percentile, 10% percentile, 25% percentile, median, 75% percentile, 90% percentile, 97.5% percentile, and maximum (Table 1). The distribution of the lymphocyte subsets could be also visualized in violin plots as demonstrated in Figure 1 A and B. In summary, the 95% reference range (from 2.5% to 97.5% percentile) for lymphocyte subset proportion was as follow: total T cells range from 47.3% to 84.5% in male and from 51.8% to 84.3% in female; helper T cells range from 21.8% to 55.4% in male and from 26.6% to 56.7% in female; cytotoxic T cells range from14.4% to 41.0% in male and from 14.9% to 37.4% in female; B cells range from 7.9% to 36.8% in male and from 7.6% to 33.2% in female; NK cells range from 2.3% to 24.1% in male and from 2.4% to 24.2% in female. In addition to the percentage, the absolute count of lymphocytes was obtained from hematology analysis, which was then used to calculate the cell count of lymphocyte subsets. The distribution of the data was illustrated in Figure 1 C and D, and the parameters of the distribution were summarized in Table 2. Specifically, the 95% reference range for the cell count of lymphocyte subsets was as follow: total lymphocytes range from 2062 to 9359 cells/ml in male and from 1795 to 7874 cells/ml in female; total T cells range from 1349 to 6571 cells/ml in male and from 1196 to 5873 cells/ml in female; helper T cells range from 748 to 3770 cells/ml in male and from 705 to 3546 cells/ml in female; cytotoxic T cells range from 403 to 2952 cells/ml in male and from 335 to 2193 cells/ml in female; B cells range from 276 to 2629 cells/ml in male and from 236 to 1728 cells/ml in female; NK cells range from 88 to 1415 cells/ml in male and from 75 to 1053 cells/ml in female.
Conclusion: Based on lymphocyte immunophenotyping data from 1138 untreated animals, a reference range was established for the percentage and the absolute count of peripheral blood lymphocyte subpopulations in cynomolgus monkey. This database is of profound significance as it could serve as a reliable reference to interpret the preclinical immunotoxicity findings.
References: 1. Kennedy L B, Salama A K S. A review of cancer immunotherapy toxicity. CA: a cancer journal for clinicians, 2020, 70(2): 86-104.
2. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721-1728.
3. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198.
4. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Nonclinical Safety Evaluation of the Immunotoxic Potential of Drugs and Biologics Guidance for Industry. 2020.
5. European Medicines Agency. ICH Topic S8 Immunotoxicity Studies for Human Pharmaceuticals. 2006.
Acknowledgments: Jinpeng Li*, Hua Yang* contributed equally to this poster
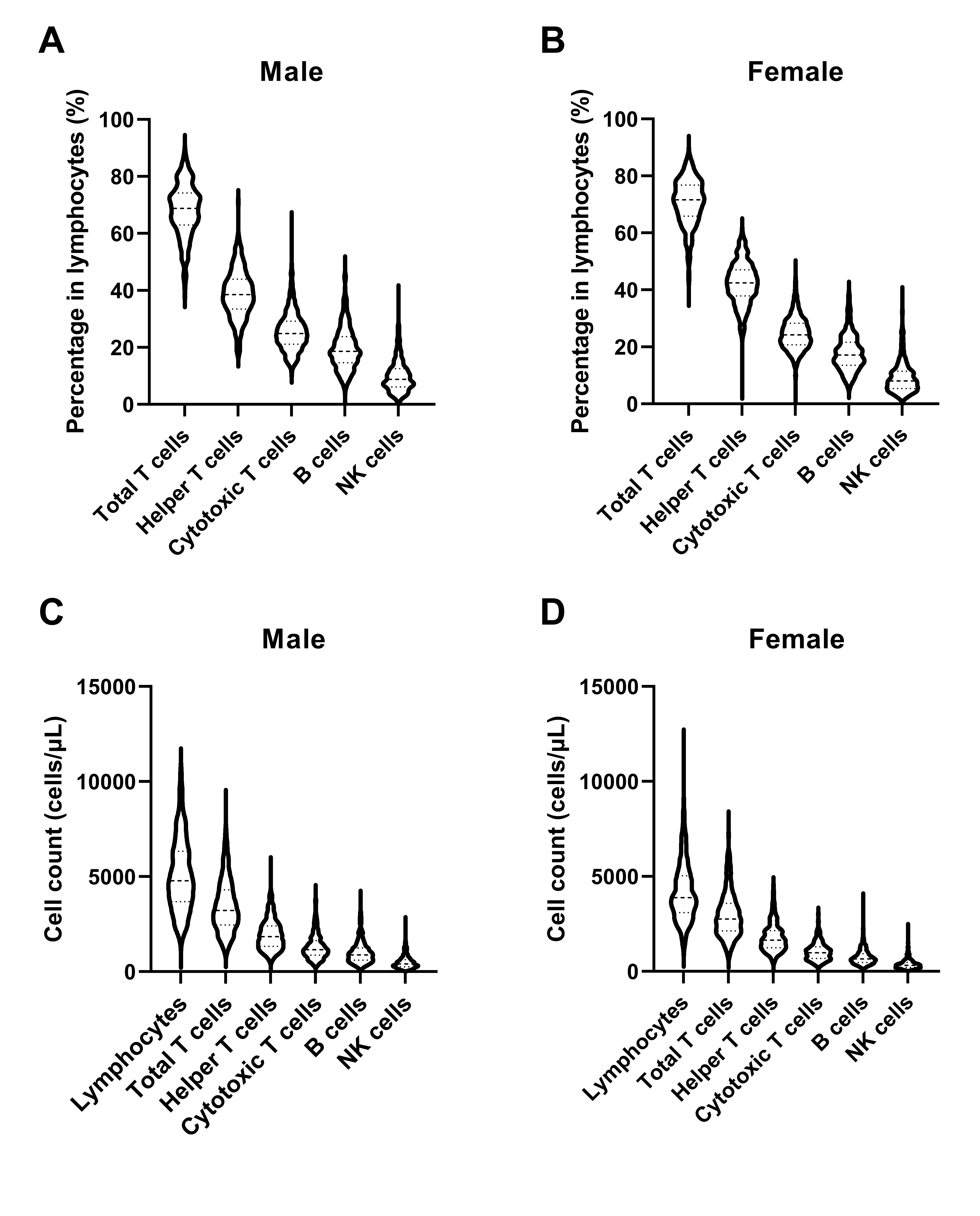
Figure 1. The distribution of the percentage (A, B) and cell count (C, D) of lymphocyte subpopulations in male (A, C) and female (B, D) cynomolgus monkey.
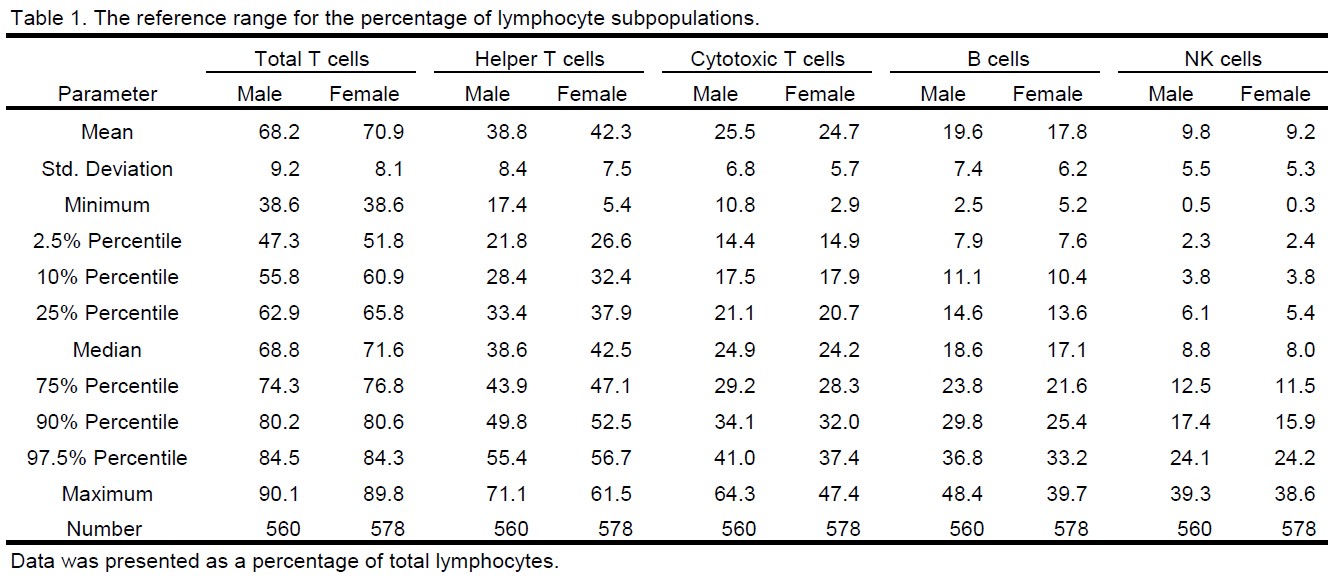
The reference range for the percentage of lymphocyte subpopulation
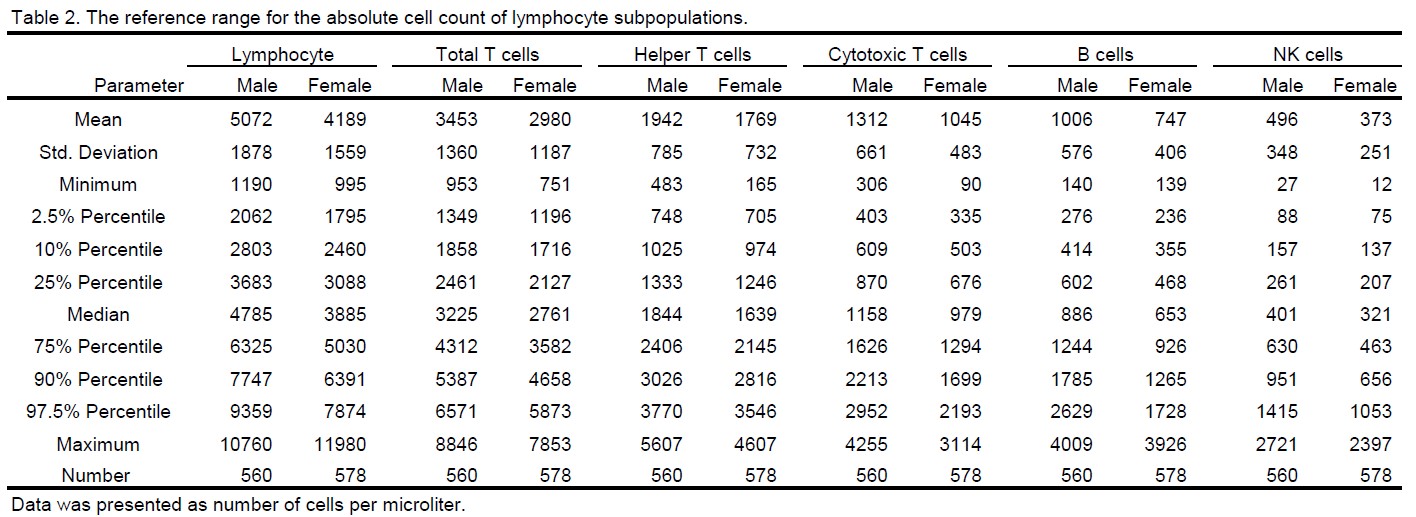
The reference range for the absolute count of lymphocyte subpopulations
Preclinical Development - Biomolecular - Safety
Category: Late Breaking Poster Abstract
(M1130-02-09) Establishment of Preclinical Immunotoxicity Reference Based on a Large-Scale Immunophenotyping Data in Cynomolgus Monkey
Monday, October 17, 2022
11:30 AM – 12:30 PM ET

Jinping Lai, PhD
Director - Large Molecules Bioanalysis
WuXi AppTec- JL
Jinping Li, Ph.D.
WuXi AppTec
Suzhou, Jiangsu, China (People's Republic)
Presenter (non-author)(s)
Main Author(s)
Purpose: Cancer immunotherapy has rapidly emerged and advanced in the past few years. The success of immunotherapy relies on delicately harnessing the power of the immune system, including modulating immune functions using cytokines, inhibiting immune checkpoints, and manipulating immune cells to recognize cancer cells (1). The immune system comprises a variety of tissues, cell types, and molecules, which are intricately connected and highly regulated. Therefore, therapeutics modulating the immune system could sometimes become double-edged swords and result in collateral damage on normal tissues. Severe and even fatal adverse effects have been reported during clinical trials of immunomodulatory therapeutics highlighting the need for assessing immunotoxicity in preclinical studies (2, 3). Currently, regulatory guidance from both Food and Drug Administration and European Medicines Agency recommend a weight-of-evidence approach to assess the immunotoxicity potential of drugs and biologics (4, 5). One of the preclinical immunotoxicity study recommended is immunophenotyping in order to identify the specific cell populations affected and to provide useful clinical biomarkers (5). Given the physiological relevance, non-human primates, especially cynomolgus monkeys, are widely used for immunotoxicological testing of pharmaceutical reagents. The objective of this report is to establish a reliable lymphocyte immunophenotyping reference range in cynomolgus monkey, which could significantly aid in the immunotoxicological assessment in preclinical study.
Methods: Animals - Cynomolgus monkeys used in this report were originated from China. All animals were naïve and maintained in compliance with the Guide for the Care and Use of Laboratory Animals. All animal research was approved by the Institutional Animal Care and Use Committee. Immunophenotyping - Blood samples were collected with anti-coagulant potassium EDTA and washed with 2 mL of DPBS by centrifugation of 5 min at 400 g. Antibody cocktail was added which includes: FITC anti-NHP CD45, V450 anti-human CD3, PerCP-Cy5.5 anti-human CD4, PE anti-human CD8, APC anti-human CD20, and BV 510 anti-human CD16. Cells were incubated with antibody cocktail at room temperature for 20 to 30 minutes protected from white light. Cells were then washed and re-suspended with DPBS and analyzed using BD LSRFortessaTM flow cytometer with FACS Diva software.
Results: Lymphocyte immunophenotyping was conducted in peripheral blood collected from 560 untreated male and 578 untreated female cynomolgus monkeys. The percentage of lymphocyte subpopulations was reported in Table 1, which includes total T cell (CD3+), helper T cell (CD3+ CD4+ CD8-), cytotoxic T cell (CD3+ CD4- CD8+), B cell (CD3- CD20+), and natural killer (NK) cell (CD3- CD16+). To assess the distribution of the data, a variety of parameters were calculated including the mean, standard deviation, minimum, 2.5% percentile, 10% percentile, 25% percentile, median, 75% percentile, 90% percentile, 97.5% percentile, and maximum (Table 1). The distribution of the lymphocyte subsets could be also visualized in violin plots as demonstrated in Figure 1 A and B. In summary, the 95% reference range (from 2.5% to 97.5% percentile) for lymphocyte subset proportion was as follow: total T cells range from 47.3% to 84.5% in male and from 51.8% to 84.3% in female; helper T cells range from 21.8% to 55.4% in male and from 26.6% to 56.7% in female; cytotoxic T cells range from14.4% to 41.0% in male and from 14.9% to 37.4% in female; B cells range from 7.9% to 36.8% in male and from 7.6% to 33.2% in female; NK cells range from 2.3% to 24.1% in male and from 2.4% to 24.2% in female. In addition to the percentage, the absolute count of lymphocytes was obtained from hematology analysis, which was then used to calculate the cell count of lymphocyte subsets. The distribution of the data was illustrated in Figure 1 C and D, and the parameters of the distribution were summarized in Table 2. Specifically, the 95% reference range for the cell count of lymphocyte subsets was as follow: total lymphocytes range from 2062 to 9359 cells/ml in male and from 1795 to 7874 cells/ml in female; total T cells range from 1349 to 6571 cells/ml in male and from 1196 to 5873 cells/ml in female; helper T cells range from 748 to 3770 cells/ml in male and from 705 to 3546 cells/ml in female; cytotoxic T cells range from 403 to 2952 cells/ml in male and from 335 to 2193 cells/ml in female; B cells range from 276 to 2629 cells/ml in male and from 236 to 1728 cells/ml in female; NK cells range from 88 to 1415 cells/ml in male and from 75 to 1053 cells/ml in female.
Conclusion: Based on lymphocyte immunophenotyping data from 1138 untreated animals, a reference range was established for the percentage and the absolute count of peripheral blood lymphocyte subpopulations in cynomolgus monkey. This database is of profound significance as it could serve as a reliable reference to interpret the preclinical immunotoxicity findings.
References: 1. Kennedy L B, Salama A K S. A review of cancer immunotherapy toxicity. CA: a cancer journal for clinicians, 2020, 70(2): 86-104.
2. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721-1728.
3. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198.
4. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Nonclinical Safety Evaluation of the Immunotoxic Potential of Drugs and Biologics Guidance for Industry. 2020.
5. European Medicines Agency. ICH Topic S8 Immunotoxicity Studies for Human Pharmaceuticals. 2006.
Acknowledgments: Jinpeng Li*, Hua Yang* contributed equally to this poster

Figure 1. The distribution of the percentage (A, B) and cell count (C, D) of lymphocyte subpopulations in male (A, C) and female (B, D) cynomolgus monkey.

The reference range for the percentage of lymphocyte subpopulation

The reference range for the absolute count of lymphocyte subpopulations
