Back
Purpose: The aim of this work is to investigate the suitability of two newly developed synthetic membranes, comprising biomimetic barriers (named Permeapad™ and SCM) for drug permeability by comparison with human skin.
Methods: The permeability of two donor human skin was compared using these two synthetic membranes. Transdermal formulations of Levonorgestrel (LNG) and Ethinyl Estradiol (EE) were used for this study. The form of transdermal path is the adhesive matrix or drug-in-adhesive (DIA) design. The permeation studies were investigated for a period of 7 days using vertical Franz Cells.
Results: The result demonstrated that, for Levonorgestrel, PermeaPad has the least variation than other two (Human skin > SCM > Permeapad) for LNG. In addition, the permeability of SCM was closer to that of human skin (Permeapad > SCM > Human skin). For Ethinyl Estradiol, there was not any permeation observed by using Permeapad. However, the permeation profile was very close to the human skin by using SCM. Both barriers have little batch to batch variation.
Conclusion: From our results of permeation study of LNG and EE, SCM that contained of the lipophilic extract of the plant Zingiber officinale Roscoe has less variation than skin but closer permeability to skin and is a better substitute for human skin than Permeapad.
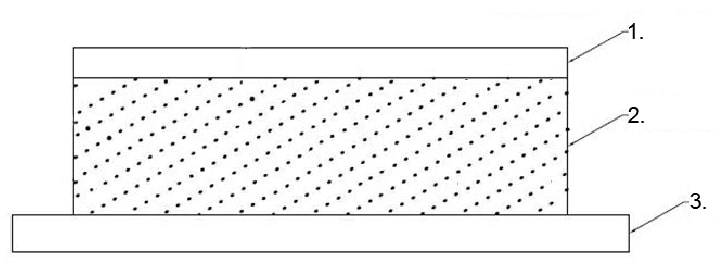
Figure 1. Drug-in-adhesive (DIA) or adhesive matrix type transdermal patch in which the backing film (1) is affixed atop the adhesive drug matrix (2), which is supported by an over-sized release liner film (3).
.jpg)
Figure 2. Comparison of 7 days permeation for LNG formulation through human skin, SCM and Permeapad.
.jpg)
Figure 3. Comparison of 7 days permeation for EE formulation through human skin and SCM
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1030-01-03) In Vitro Permeability Comparison Study: Skin versus Two Biomimetic Barrier Permeapad™ and SCM
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- ML
Minghao Li, MS
Logan Instruments
Somerset, New Jersey, United States - IT
Ichuang Tan, BS
Bionex Pharmaceuticals LLC
North Brunswick, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: The aim of this work is to investigate the suitability of two newly developed synthetic membranes, comprising biomimetic barriers (named Permeapad™ and SCM) for drug permeability by comparison with human skin.
Methods: The permeability of two donor human skin was compared using these two synthetic membranes. Transdermal formulations of Levonorgestrel (LNG) and Ethinyl Estradiol (EE) were used for this study. The form of transdermal path is the adhesive matrix or drug-in-adhesive (DIA) design. The permeation studies were investigated for a period of 7 days using vertical Franz Cells.
Results: The result demonstrated that, for Levonorgestrel, PermeaPad has the least variation than other two (Human skin > SCM > Permeapad) for LNG. In addition, the permeability of SCM was closer to that of human skin (Permeapad > SCM > Human skin). For Ethinyl Estradiol, there was not any permeation observed by using Permeapad. However, the permeation profile was very close to the human skin by using SCM. Both barriers have little batch to batch variation.
Conclusion: From our results of permeation study of LNG and EE, SCM that contained of the lipophilic extract of the plant Zingiber officinale Roscoe has less variation than skin but closer permeability to skin and is a better substitute for human skin than Permeapad.

Figure 1. Drug-in-adhesive (DIA) or adhesive matrix type transdermal patch in which the backing film (1) is affixed atop the adhesive drug matrix (2), which is supported by an over-sized release liner film (3).
.jpg)
Figure 2. Comparison of 7 days permeation for LNG formulation through human skin, SCM and Permeapad.
.jpg)
Figure 3. Comparison of 7 days permeation for EE formulation through human skin and SCM
