Back
Purpose: Acyclovir is an antiviral drug primarily used to treat herpes simplex virus infections, chickenpox, and shingles [1]. Acyclovir has low aqueous solubility and oral bioavailability [2]. The main objective of this study was to enhance the solubility of Acyclovir by amorphous solid dispersions using polymeric biomaterials with hot melt extrusion technology. Selecting a suitable polymeric biomaterial can provide thermodynamic stability to a metastable form with higher solubility. This solubility enhancement to the active pharmaceutical ingredient may ensure the safety and efficacy of the formulation [3].
Methods: A High-Performance Liquid Chromatography (HPLC) method for quantification of Acyclovir was developed and validated as per USP guidelines. Shimadzu Prominence-i LC-2030C equipped with PDA detector and Phenomenex C-18 (150 × 4.6 mm) column was used. The mobile phase consisted of acetonitrile and water in the ratio of 50:50 (v/v) at a flow rate of 1mL/min; the concentration range of the standard curve was 2 to 25 µg/mL. Analysis was carried out at the λmax of 253 nm. Amorphous solid dispersions of Acyclovir with four different polymers (Kollidon VA 64, Soluplus, HPMC, and Eudragit E100) were prepared using the hot melt extrusion equipment (HAAKE Mini CTW, ThermoScientific). The appropriate extrusion temperatures were set by analyzing the differential scanning calorimetry (DSC) curves and thermogravimetric analysis (TGA) of the polymers and the drug. The extrudates were characterized after trituration in a grinder. DSC, Fourier transformed infrared spectroscopy (FTIR), and Scanning electron microscopy (SEM) were used to characterize the extrudates. The HPLC method was used to determine the 24 hours saturation solubility of Acyclovir and its solid dispersions.
Results: The standard curve for Acyclovir in the HPLC method was linear over the concentration ranges studied with R2≥0.999. The method was specific for Acyclovir, and the retention time of the drug was 1.6 ±0.05 min. Intra-day (n=4) and Inter-day (n=6) analytical precision and accuracy for Acyclovir were within the USP limits. From the analysis of Acyclovir and Kollidon VA 64 DSC data, the melting point of Acyclovir was found to be 259°C, and the glass transition temperature for Kollidon VA 64 was found to be 106°C to prevent drug degradation and maintain polymer viscosity while extrusion, the hot melt extrusion temperature was set to 180°C at 150 RPM. Kollidon VA 64 was selected as the most suitable polymer because the solid dispersion of Acyclovir with Kollidon VA 64 showed amorphous characteristics in DSC (Figure 1), which was also evident from SEM. The FTIR data for Acyclovir solid dispersions with Kollidon VA 64 showed no noticeable change in the molecular characteristics of the drug (Figure 2). From the saturation solubility studies (Figure 3) of three different solid dispersion formulations of Acyclovir with Kollidon VA 64 in the ratios 1:4 (w/w), 1.5:3.5 (w/w), and 2:3 (w/w), it was found that all three formulations showed significant enhancement in solubility (p < 0.001) with ACY: Kollidon VA 64 (1.5:3.5) showing maximum enhancement in solubility followed by ACY: Kollidon VA 64 (1:4) and ACY: Kollidon VA 64 (2:3).
Conclusion: The HPLC method was developed and validated as per USP guidelines. Solid dispersion formulations of Acyclovir were developed using polymeric biomaterials, and the drug was found to present in the amorphous form with no noticeable changes in molecular characteristics; this was confirmed by DSC, SEM, and FTIR studies. Based on the saturation solubility studies, formulation with Acyclovir and Kollidon VA64 in the ratio of 1.5:3.5 showed the most significant improvement in the solubility of Acyclovir.
References: 1. Taylor, Michael, and Valerie Gerriets. 2022 "Acyclovir." in StatPearls Treasure Island (FL): StatPearls Publishing.
2. Savjani, Jignasa, and Chirag Pathak, 2016. Improvement of Physicochemical Parameters of Acyclovir Using Cocrystallization Approach. Brazilian Journal of Pharmaceutical Sciences 52:727-34. doi: 10.1590/s1984-82502016000400017.
3. Baghel, Shrawan, Helen Cathcart, and Niall J. O'Reilly, 2016. "Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. Journal of Pharmaceutical Sciences 105(9):2527-44. doi: 10.1016/j.xphs.2015.10.008.
Acknowledgments: The authors thank Dr. Joel Destino, Assistant Professor, College of Arts and Sciences, Creighton University, for SEM imaging.
Funding: The authors gratefully thank the Department of Pharmacy Sciences, Creighton University, for the financial support.
Conflict of Interest Statements: We confirm that there are no conflicts of interest associated with this work, and there has been no significant financial support for this work that could have influenced its outcome.
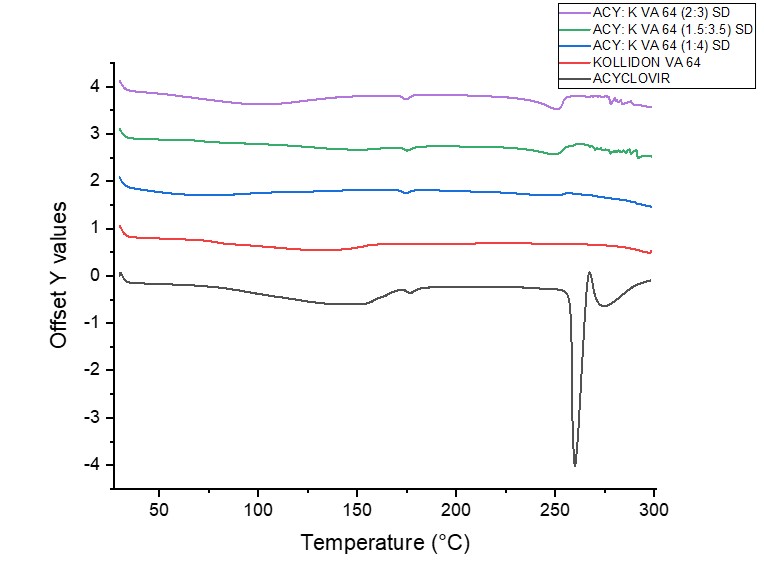
Figure 1: DSC thermograms of Acyclovir, Kollidon VA 64, and solid dispersions of Acyclovir and Kollidon VA 64 in the ratios 1:4 (w/w), 1.5:3.5 (w/w), and 2:3 (w/w), respectively.
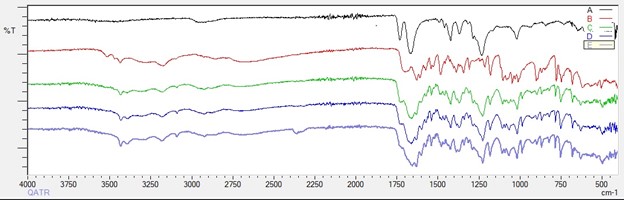
Figure 2: FTIR Spectra of – Kollidon VA 64 (A), Acyclovir (B), ACY: Kollidon VA 64 SD 1:4 (w/w) (C), ACY: Kollidon VA 64 SD 1.5:3.5 (w/w) (D), and ACY: Kollidon VA 64 SD 2:3 (w/w) (E).
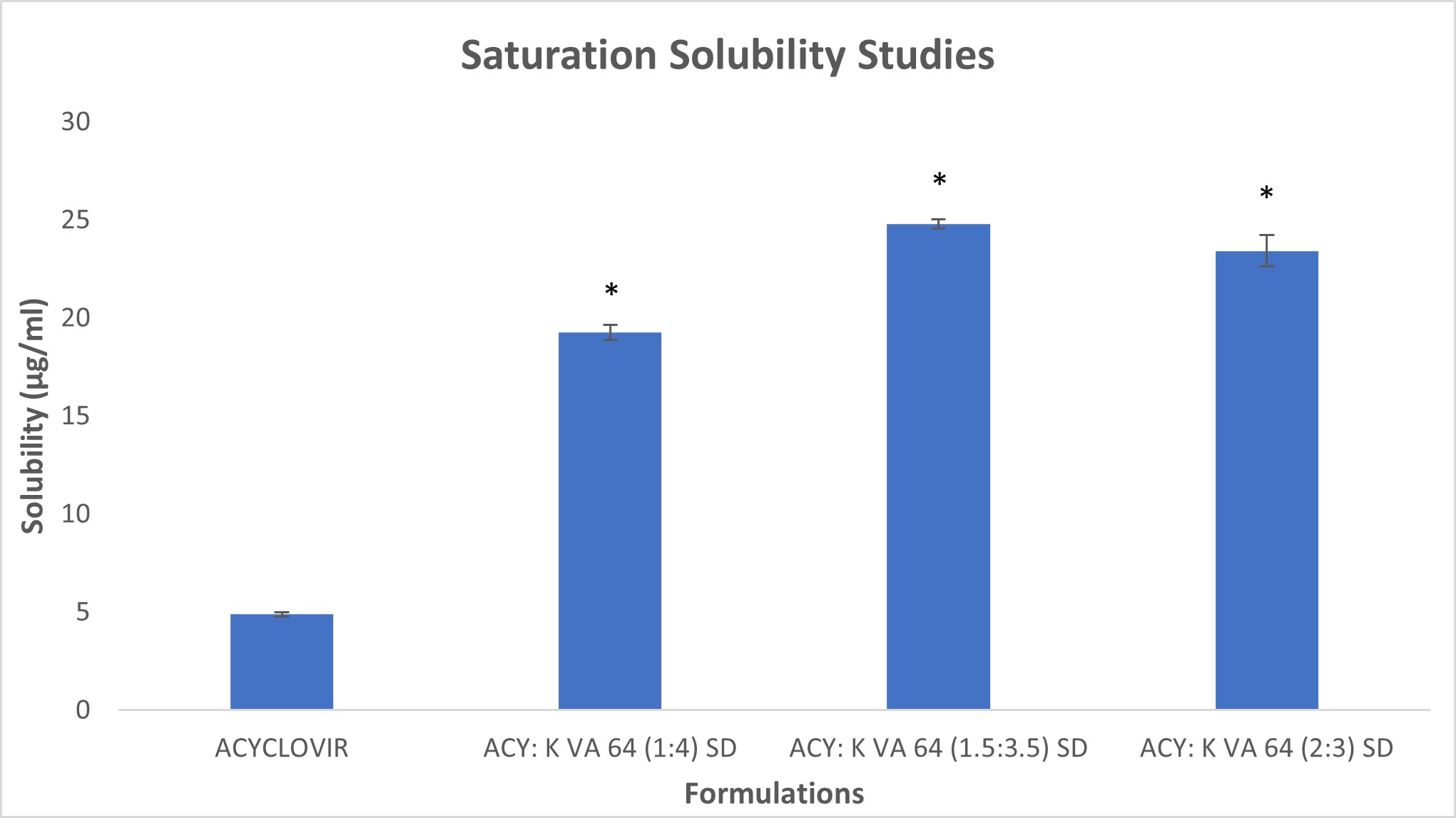
Figure 3: Saturation solubility of Acyclovir in the free form and the Kollidon VA 64 solid dispersions with the asterisk representing the level of significance compared to Acyclovir (*p < 0.001)
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1030-12-70) Solubility Enhancement of BCS Class IV Drug Acyclovir Using Hot Melt Extrusion
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- PR
Pankaj Rajdeo, BS
Creighton University
Omaha, Nebraska, United States - PR
Pankaj Rajdeo, BS
Creighton University
Omaha, Nebraska, United States
Presenting Author(s)
Main Author(s)
Purpose: Acyclovir is an antiviral drug primarily used to treat herpes simplex virus infections, chickenpox, and shingles [1]. Acyclovir has low aqueous solubility and oral bioavailability [2]. The main objective of this study was to enhance the solubility of Acyclovir by amorphous solid dispersions using polymeric biomaterials with hot melt extrusion technology. Selecting a suitable polymeric biomaterial can provide thermodynamic stability to a metastable form with higher solubility. This solubility enhancement to the active pharmaceutical ingredient may ensure the safety and efficacy of the formulation [3].
Methods: A High-Performance Liquid Chromatography (HPLC) method for quantification of Acyclovir was developed and validated as per USP guidelines. Shimadzu Prominence-i LC-2030C equipped with PDA detector and Phenomenex C-18 (150 × 4.6 mm) column was used. The mobile phase consisted of acetonitrile and water in the ratio of 50:50 (v/v) at a flow rate of 1mL/min; the concentration range of the standard curve was 2 to 25 µg/mL. Analysis was carried out at the λmax of 253 nm. Amorphous solid dispersions of Acyclovir with four different polymers (Kollidon VA 64, Soluplus, HPMC, and Eudragit E100) were prepared using the hot melt extrusion equipment (HAAKE Mini CTW, ThermoScientific). The appropriate extrusion temperatures were set by analyzing the differential scanning calorimetry (DSC) curves and thermogravimetric analysis (TGA) of the polymers and the drug. The extrudates were characterized after trituration in a grinder. DSC, Fourier transformed infrared spectroscopy (FTIR), and Scanning electron microscopy (SEM) were used to characterize the extrudates. The HPLC method was used to determine the 24 hours saturation solubility of Acyclovir and its solid dispersions.
Results: The standard curve for Acyclovir in the HPLC method was linear over the concentration ranges studied with R2≥0.999. The method was specific for Acyclovir, and the retention time of the drug was 1.6 ±0.05 min. Intra-day (n=4) and Inter-day (n=6) analytical precision and accuracy for Acyclovir were within the USP limits. From the analysis of Acyclovir and Kollidon VA 64 DSC data, the melting point of Acyclovir was found to be 259°C, and the glass transition temperature for Kollidon VA 64 was found to be 106°C to prevent drug degradation and maintain polymer viscosity while extrusion, the hot melt extrusion temperature was set to 180°C at 150 RPM. Kollidon VA 64 was selected as the most suitable polymer because the solid dispersion of Acyclovir with Kollidon VA 64 showed amorphous characteristics in DSC (Figure 1), which was also evident from SEM. The FTIR data for Acyclovir solid dispersions with Kollidon VA 64 showed no noticeable change in the molecular characteristics of the drug (Figure 2). From the saturation solubility studies (Figure 3) of three different solid dispersion formulations of Acyclovir with Kollidon VA 64 in the ratios 1:4 (w/w), 1.5:3.5 (w/w), and 2:3 (w/w), it was found that all three formulations showed significant enhancement in solubility (p < 0.001) with ACY: Kollidon VA 64 (1.5:3.5) showing maximum enhancement in solubility followed by ACY: Kollidon VA 64 (1:4) and ACY: Kollidon VA 64 (2:3).
Conclusion: The HPLC method was developed and validated as per USP guidelines. Solid dispersion formulations of Acyclovir were developed using polymeric biomaterials, and the drug was found to present in the amorphous form with no noticeable changes in molecular characteristics; this was confirmed by DSC, SEM, and FTIR studies. Based on the saturation solubility studies, formulation with Acyclovir and Kollidon VA64 in the ratio of 1.5:3.5 showed the most significant improvement in the solubility of Acyclovir.
References: 1. Taylor, Michael, and Valerie Gerriets. 2022 "Acyclovir." in StatPearls Treasure Island (FL): StatPearls Publishing.
2. Savjani, Jignasa, and Chirag Pathak, 2016. Improvement of Physicochemical Parameters of Acyclovir Using Cocrystallization Approach. Brazilian Journal of Pharmaceutical Sciences 52:727-34. doi: 10.1590/s1984-82502016000400017.
3. Baghel, Shrawan, Helen Cathcart, and Niall J. O'Reilly, 2016. "Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. Journal of Pharmaceutical Sciences 105(9):2527-44. doi: 10.1016/j.xphs.2015.10.008.
Acknowledgments: The authors thank Dr. Joel Destino, Assistant Professor, College of Arts and Sciences, Creighton University, for SEM imaging.
Funding: The authors gratefully thank the Department of Pharmacy Sciences, Creighton University, for the financial support.
Conflict of Interest Statements: We confirm that there are no conflicts of interest associated with this work, and there has been no significant financial support for this work that could have influenced its outcome.

Figure 1: DSC thermograms of Acyclovir, Kollidon VA 64, and solid dispersions of Acyclovir and Kollidon VA 64 in the ratios 1:4 (w/w), 1.5:3.5 (w/w), and 2:3 (w/w), respectively.

Figure 2: FTIR Spectra of – Kollidon VA 64 (A), Acyclovir (B), ACY: Kollidon VA 64 SD 1:4 (w/w) (C), ACY: Kollidon VA 64 SD 1.5:3.5 (w/w) (D), and ACY: Kollidon VA 64 SD 2:3 (w/w) (E).

Figure 3: Saturation solubility of Acyclovir in the free form and the Kollidon VA 64 solid dispersions with the asterisk representing the level of significance compared to Acyclovir (*p < 0.001)
