Back
Purpose: In recent years, the need to manufacture larger batch sizes to accommodate increased demand of biopharmaceutical drug development has grown significantly. This is particularly prevalent in the on-going effort to supply vaccinations to combat the current pandemic. An effect of this practice is the increased buffer consumption required to run downstream unit operations, which has in turn affected labor requirements for buffer preparation, as well as footprint for storage tanks. One solution to overcome this growing issue is the use of in-line dilution systems to produce in specification process buffer, on demand, from concentrated buffer stocks.
The successful implementation of an in-line dilution process is reliant upon three major aspects. Firstly, the development of optimized concentrated buffer stocks. Secondly, determining suitable in-line dilution flow rate ranges to meet the expected downstream buffer demands. Thirdly, an accurate and repeatable control mechanism for in-line dilution to ensure the in-line diluted process buffer meets process specifications. Work has been completed using the Allegro™ Connect Buffer Management System (BMS) to optimize each part of an automated buffer delivery process using novel flow-path configurations and biocontainer level-sensing technology.
Methods: For this work, three process buffers were chosen for in-line dilution studies: 0.1 M Glycine-HCl pH 3, 0.01 M Tris-HCl, 0.05 M NaCl pH 8 and 0.01 M Sodium phosphate, 0.05 M NaCl pH 7. Theoretical predictions based on the pKa were completed to assess the concentrate pH required for buffer stocks from 5× to 20× concentration. Concentration factors studied included the glycine-based buffer at 5× and 10×, the tris-based buffer at 10× and 20× and the phosphate-based buffer at 5× and 20×. The buffer concentrates were first optimized at benchtop scale (1 L), initially titrating as per the pH predictions based on buffer salt pKa. Benchtop dilutions were subsequently completed on each concentrate studied to, firstly, assess optimization and, secondly, determine the expected concentrate / process buffer conductivity and pH set-points for in-line dilution studies. The benchtop process was then scaled up to manufacturing scale to assess the accuracy and capacity of in-line dilution over multiple concentrate production batches, using varied in-line dilution control systems, with all six concentrate buffers installed and connected to one single-use flow path. The BMS controls in-line dilution of buffer concentrates based on a feedback loop between the concentrate pump and the in-line conductivity sensor. The in-line pH sensor acts as a reference to either accept or reject the buffer dilution but has no impact on the control loop. All six optimized concentrate buffers were produced in quantities of 60 – 100 L (concentrate/ process biocontainer size = 100 L) per batch and the in-line dilution accuracy was assessed over eight batches. Six runs were conducted using constant speed and conductivity control, with in-line dilution flow rates ranging from ~ 1000 – 1450 L/h based on required dilution factor. Two further runs were conducted with level and conductivity control with in-line dilution flow rates ranging from 400 L/h to 1450 L/h, where the system varied the in-line dilution flow rate to match the downstream draw flow rate. For each run, a sample was taken for off-line conductivity and pH measurements.
Results: Each process buffer was successfully in-line diluted with average maxima deviations of ± 0.12 and ± 1.35% from the pH and conductivity set-points, respectively, for constant speed control; and ± 0.05 and ± 0.37%, respectively, for level control. This was affirmed from offline sample testings which measured within average maxima deviations of ± 0.06 and ± 3.44% from the pH and conductivity set-points, respectively, thus showing that each concentrate was suitably optimized for the desired process buffer pH. The maximum flow rates achieved for each of the dilutions studied, namely, 5×, 10× and 20×, were measured to be ~1050 L/h, 1450 L/h and 1360 L/h, respectively, showing suitable process scalability. Additionally, the process was able to stabilize the in-line dilution within 12.2 seconds for 5× dilution factors, and 40 seconds for 10× and 20× dilution factors, resulting in minimal concentrate loss ( < 1.6 L).
Conclusion: In conclusion, the process optimization study was suitable for connection of the BMS to a downstream unit operation with the following process specifications: maximum downstream draw flow rates of 1200 L/h for 10× and 20× dilution factors and 1000 L/h for 5× dilution factors; process buffers with pH and conductivity acceptance criteria within ± 0.12 and ± 3 % respectively; and a maximum process buffer need of approximately 10,000 L based on in-line dilution from 600 L (up to six different concentrates) of 20× concentrate diluted by level control. This replaces the need of up to 10,000 L worth of storage tank footprint and manufacturing labor with the space and operator management required for just 600 L of buffer concentrates and 600 L of process buffers.
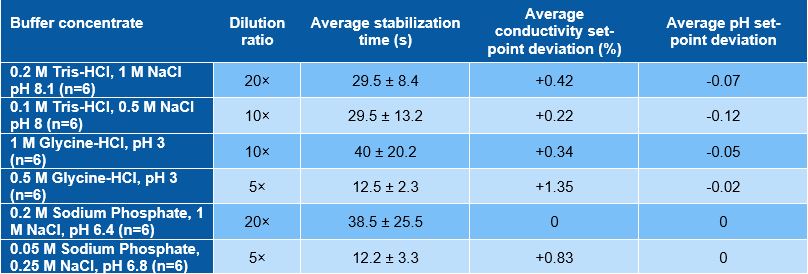
In-line dilution results summary showing the average stabilization time, conductivity set-point deviation and pH set-point deviation over the six buffers studied
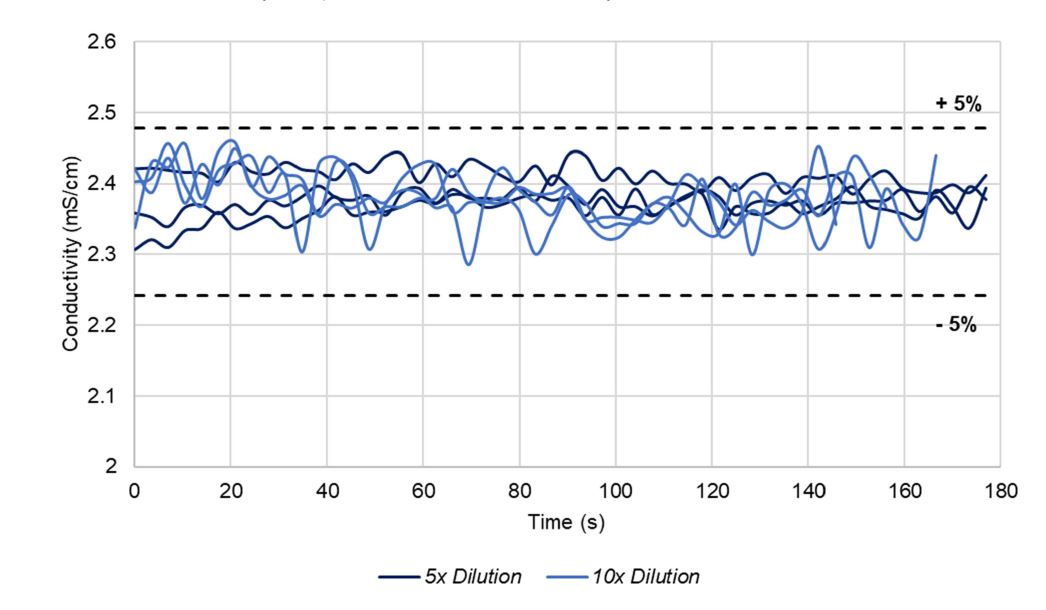
Conductivity measurements during biocontainer filling at constant speed with triplicate in-line dilutions of 1 M Glycine-HCl, pH 3.0 by 10x at ~1360 L/h and 0.5 M Glycine-HCl pH 3.0 by 5x at ~1080 L/h. Conductivity acceptance tolerances are shown by the black dashed lines.
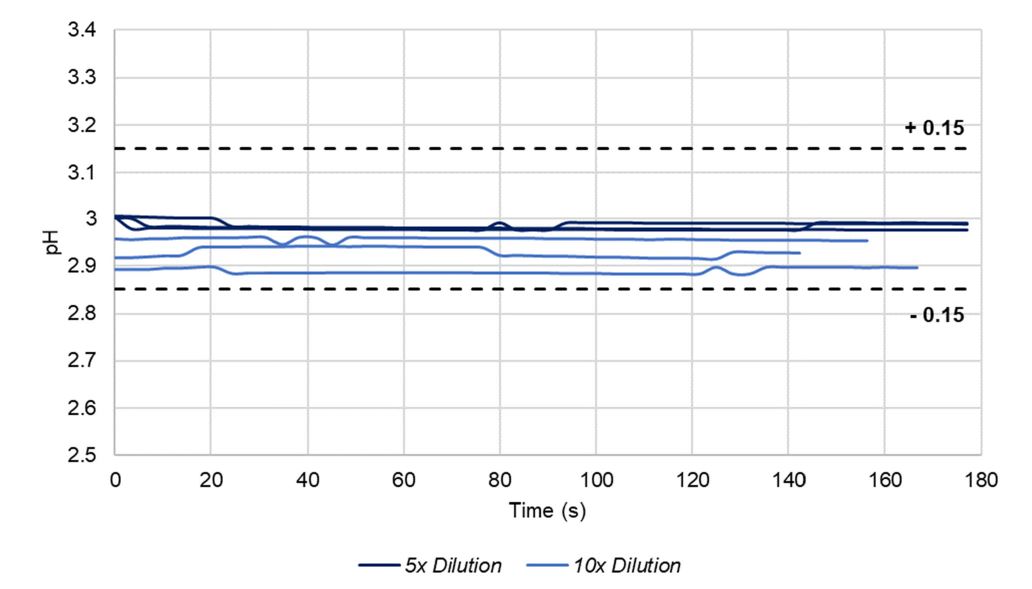
pH measurements during biocontainer filling at constant speed with triplicate in-line dilutions of 1 M Glycine-HCl, pH 3.0 by 10x at ~1360 L/h and 0.5 M Glycine-HCl pH 3.0 by 5x at ~1080 L/h. pH acceptance tolerances are shown by the black dashed lines.
Manufacturing and Analytical Characterization - Biomolecular - Single-Use and Disposable Systems
Category: Poster Abstract
(W1030-08-44) Automated and Centralized Buffer Management for Downstream Processing in Biopharmaceutical Manufacture
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- TJ
Terese Joseph
Pall Corporation
- NK
Natasha Kelly, MS
Pall Corporation
PORTSMOUTH, England, United Kingdom
Presenter (non-author)(s)
Main Author(s)
Purpose: In recent years, the need to manufacture larger batch sizes to accommodate increased demand of biopharmaceutical drug development has grown significantly. This is particularly prevalent in the on-going effort to supply vaccinations to combat the current pandemic. An effect of this practice is the increased buffer consumption required to run downstream unit operations, which has in turn affected labor requirements for buffer preparation, as well as footprint for storage tanks. One solution to overcome this growing issue is the use of in-line dilution systems to produce in specification process buffer, on demand, from concentrated buffer stocks.
The successful implementation of an in-line dilution process is reliant upon three major aspects. Firstly, the development of optimized concentrated buffer stocks. Secondly, determining suitable in-line dilution flow rate ranges to meet the expected downstream buffer demands. Thirdly, an accurate and repeatable control mechanism for in-line dilution to ensure the in-line diluted process buffer meets process specifications. Work has been completed using the Allegro™ Connect Buffer Management System (BMS) to optimize each part of an automated buffer delivery process using novel flow-path configurations and biocontainer level-sensing technology.
Methods: For this work, three process buffers were chosen for in-line dilution studies: 0.1 M Glycine-HCl pH 3, 0.01 M Tris-HCl, 0.05 M NaCl pH 8 and 0.01 M Sodium phosphate, 0.05 M NaCl pH 7. Theoretical predictions based on the pKa were completed to assess the concentrate pH required for buffer stocks from 5× to 20× concentration. Concentration factors studied included the glycine-based buffer at 5× and 10×, the tris-based buffer at 10× and 20× and the phosphate-based buffer at 5× and 20×. The buffer concentrates were first optimized at benchtop scale (1 L), initially titrating as per the pH predictions based on buffer salt pKa. Benchtop dilutions were subsequently completed on each concentrate studied to, firstly, assess optimization and, secondly, determine the expected concentrate / process buffer conductivity and pH set-points for in-line dilution studies. The benchtop process was then scaled up to manufacturing scale to assess the accuracy and capacity of in-line dilution over multiple concentrate production batches, using varied in-line dilution control systems, with all six concentrate buffers installed and connected to one single-use flow path. The BMS controls in-line dilution of buffer concentrates based on a feedback loop between the concentrate pump and the in-line conductivity sensor. The in-line pH sensor acts as a reference to either accept or reject the buffer dilution but has no impact on the control loop. All six optimized concentrate buffers were produced in quantities of 60 – 100 L (concentrate/ process biocontainer size = 100 L) per batch and the in-line dilution accuracy was assessed over eight batches. Six runs were conducted using constant speed and conductivity control, with in-line dilution flow rates ranging from ~ 1000 – 1450 L/h based on required dilution factor. Two further runs were conducted with level and conductivity control with in-line dilution flow rates ranging from 400 L/h to 1450 L/h, where the system varied the in-line dilution flow rate to match the downstream draw flow rate. For each run, a sample was taken for off-line conductivity and pH measurements.
Results: Each process buffer was successfully in-line diluted with average maxima deviations of ± 0.12 and ± 1.35% from the pH and conductivity set-points, respectively, for constant speed control; and ± 0.05 and ± 0.37%, respectively, for level control. This was affirmed from offline sample testings which measured within average maxima deviations of ± 0.06 and ± 3.44% from the pH and conductivity set-points, respectively, thus showing that each concentrate was suitably optimized for the desired process buffer pH. The maximum flow rates achieved for each of the dilutions studied, namely, 5×, 10× and 20×, were measured to be ~1050 L/h, 1450 L/h and 1360 L/h, respectively, showing suitable process scalability. Additionally, the process was able to stabilize the in-line dilution within 12.2 seconds for 5× dilution factors, and 40 seconds for 10× and 20× dilution factors, resulting in minimal concentrate loss ( < 1.6 L).
Conclusion: In conclusion, the process optimization study was suitable for connection of the BMS to a downstream unit operation with the following process specifications: maximum downstream draw flow rates of 1200 L/h for 10× and 20× dilution factors and 1000 L/h for 5× dilution factors; process buffers with pH and conductivity acceptance criteria within ± 0.12 and ± 3 % respectively; and a maximum process buffer need of approximately 10,000 L based on in-line dilution from 600 L (up to six different concentrates) of 20× concentrate diluted by level control. This replaces the need of up to 10,000 L worth of storage tank footprint and manufacturing labor with the space and operator management required for just 600 L of buffer concentrates and 600 L of process buffers.

In-line dilution results summary showing the average stabilization time, conductivity set-point deviation and pH set-point deviation over the six buffers studied

Conductivity measurements during biocontainer filling at constant speed with triplicate in-line dilutions of 1 M Glycine-HCl, pH 3.0 by 10x at ~1360 L/h and 0.5 M Glycine-HCl pH 3.0 by 5x at ~1080 L/h. Conductivity acceptance tolerances are shown by the black dashed lines.

pH measurements during biocontainer filling at constant speed with triplicate in-line dilutions of 1 M Glycine-HCl, pH 3.0 by 10x at ~1360 L/h and 0.5 M Glycine-HCl pH 3.0 by 5x at ~1080 L/h. pH acceptance tolerances are shown by the black dashed lines.
