Back
Purpose: For many novel antibiotics in the preclinical pipeline, as well as other novel compounds, sufficient oral bioavailability (BA) represents a critical parameter in preclinical drug development. Corallopyronin A (CorA), currently in preclinical drug development, is an antibiotic with activity against Gram positive and Gram negative pathogens including Wolbachia, endosymbionts of filarial nematodes2, Staphylococcus aureus and Chlamydia trachomatis. As a highly permeable, but pH dependent soluble acidic compound, the absorption of CorA (pKa 3.7; logP 5.4)1 is dependent on intestinal pH conditions. The objective of the present study was to elucidate the BA of CorA and two solubility enhanced CorA amorphous-solid-dispersions (ASD). The species mouse is often used as a preclinical efficacy model due to its lower material need, availability of various knock-out strains and an ease of handling. This makes early formulation development all the more important in order to provide a formulation with enhanced solubility and bioavailability already for preclinical animal species. Despite the high pharmacodynamic relevance of these models, oral pharmacokinetic testing remains challenging. Therefore, in vitro methods tailored to the respective species combined with physiologically-based PK-modeling (PBPK) were applied to support future formulation optimization and potentially inter species performance predictions.
Methods: In Vitro Biphasic and Monophasic Dissolution. Neat CorA and CorA-ASD formulations were investigated using the biphasic dissolution apparatus BiPHa+3. In order to mimic biorelevant conditions the experimental design was adapted to the in vivo used species mouse concerning the pH-profile, bile salt concentrations and transit time. Moreover, fed state was assumed to be the most appropriate condition. Figure 1 summarizes the experimental setup. The concentration profiles of the aqueous and organic phase were measured online continuously, with an Agilent 8454 UV-Vis spectrophotometer (Waldbronn, Germany) and quantified via external calibration curve at 394 nm. Likewise, the dissolution experiments were conducted without an organic phase to obtain dissolution parameters for PBPK modelling (data not shown).Pharmacokinetic Study. The in vivo studies were conducted in BALB/c mice. Neat CorA and CorA-ASD-formulations were administered orally as a water suspension, a CorA solution was administered intravenously, both with a dose of 36 mg/kg. The mice had free access to food over the entire experiment. Exposures (AUC0-inf) were measured and the oral bioavailability calculated. Physiological based pharmacokinetic modelling.Plasma concentration-time profiles for CorA-povidone and CorA-copovidone ASD formulations were simulated using the PBPK modelling software GastroPlus™ 9.8 (Simulations Plus, Inc., 2020). Data describing the physicochemical properties and the physiology of the investigated species were obtained from in vitro studies1 and from estimates calculated by ADMET predictor 9.5 (Simulations Plus, Inc., 2019). In the following, dissolution results were incorporated to the model, via Weibull-parameters calculated from a monophasic dissolution setup.
Results: In Vitro Biorelevant Dissolution and in vitro in vivo Relationship. The concentrations in the aqueous phase reached for neat CorA < 1% during the stomach simulation and 2% during the simulation of the small intestine. CorA povidone reached 78% followed by a decrease to 55% within approx. 30 minutes. For CorA-copovidone only 10% were achieved by the end of the dissolution. By using the ASD formulation approach in vitro biphasic dissolution exhibited enhanced dissolution performances. The amount of partitioned drug into the organic phase after 80 min represents the in vitro measured fraction absorbed. Neat CorA achieved only 4% partitioning, whereas for CorA-povidone 34% and for CorA-copovidone 13% could be achieved, corroborating the solubility enhancement by the formulation principle. The end point concentrations of the organic phase were correlated with the bioavailability of neat CorA and the CorA-ASD-formulations. A good correlation between both parameters was determined (R2 = 0.9966, Fig. 2). Physiological based pharmacokinetic modelling. The IV data were used to provide elimination and distribution behavior. Measured and predicted physicochemical and physiological properties were used to build a PBPK model for oral administration of CorA-ASD formulations. By incorporating the dissolution profiles of the monophasic dissolutions, plasma concentrations of the CorA-ASD-formulations were successfully predicted. Prediction errors (PE) < 15% were reached for CorA-povidone (Cmax: 1.71%; AUC0-inf: -11.31%). For CorA-copovidone Cmax and AUC0-inf were slightly overpredicted (Cmax: -16.20%; AUC0-inf: -12.43%), but still within an acceptable range (Fig. 3).
Conclusion: The mini-scale biphasic dissolution test BiPHa+ adjusted to gastric pH-levels of mice resulted in level C in vitro in vivo relationship. Moreover, determined intrinsic dissolution data in biorelevant media enabled to deduct dissolution as the decisive mechanism governing oral bioavailability of CorA and CorA-formulations. Hence, the presented setup confirmed its in vivo relevance for mice, thus providing a material saving method to optimize oral treatment of mice even in early preclinical studies. The incorporation of biorelevant dissolution into the PBPK model allowed a prediction of plasma concentration in mice. Additionally, the method can potentially be used to forecast the performance of CorA in other species via changed pH-settings of the BiPHa+ assay combined with PBPK-modelling to identify the later dose regimen for human.
References: 1. Krome, A. K. et al. Solubility and Stability Enhanced Oral Formulations for the Anti-Infective Corallopyronin A. Pharmaceutics 21, (2020).
2. Schiefer, A. et al. Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections. PLoS Negl Trop Dis 14, (2020).
3. Denninger, A., et al. A Rational Design of a Biphasic Dissolution Setup—Modelling of Biorelevant Kinetics for a Ritonavir Hot-Melt Extruded Amorphous Solid Dispersion. Pharmaceutics 12, 237 (2020).
Acknowledgements:We would like to thank the German Center for Infection Research (www.DZIF.de) grant numbers TTU 09.807, 09.816, 09.914 and the Federal Ministry of Education and Research (www.BMBF.de) grant number 16GW0229 for funding.
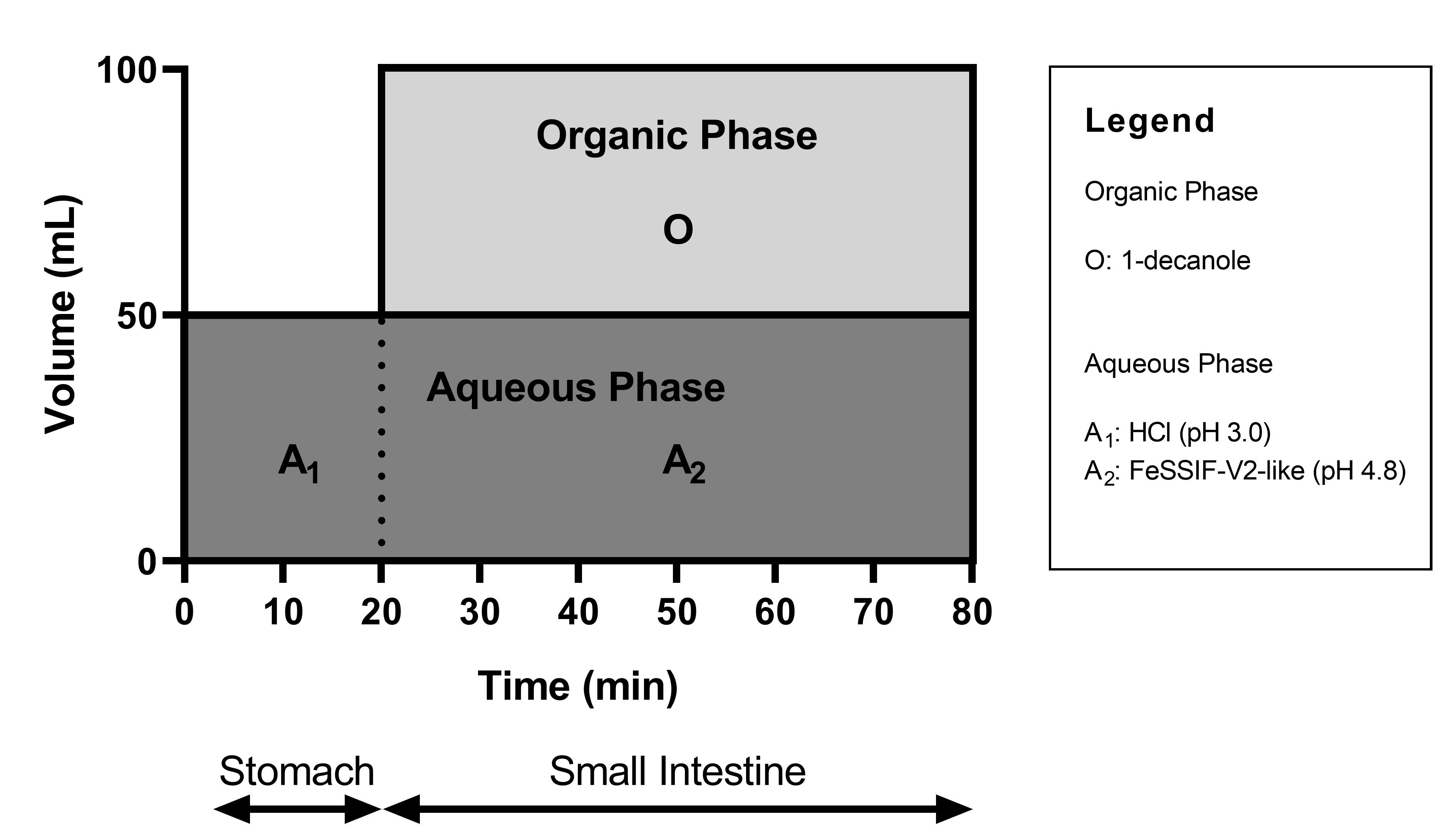
Biphasic dissolution setup for the mouse model.
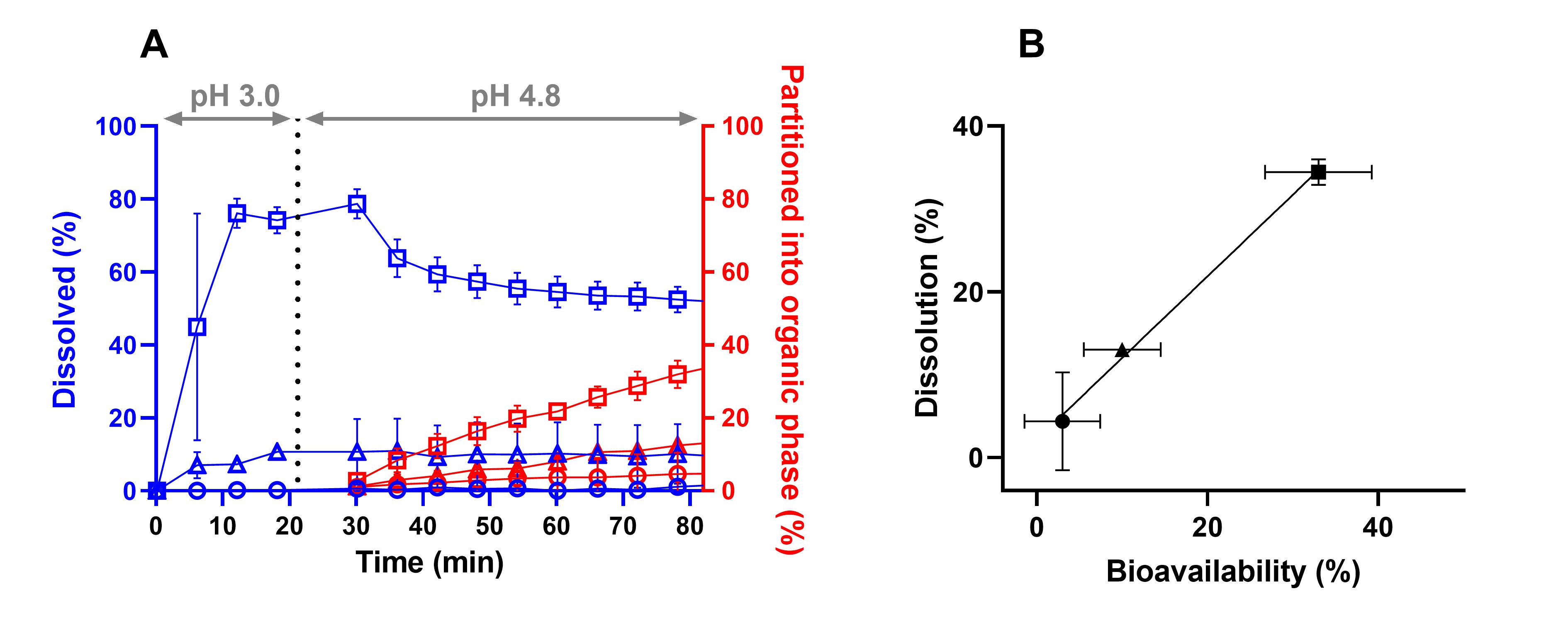
A: Biphasic dissolution under mouse gut conditions; B: Level C in vitro in vivo relationship: In vitro partitioned drug into the organic phase and in vivo BA; neat CorA (●), CorA-povidone (■) and CorA-copovidone (▲).
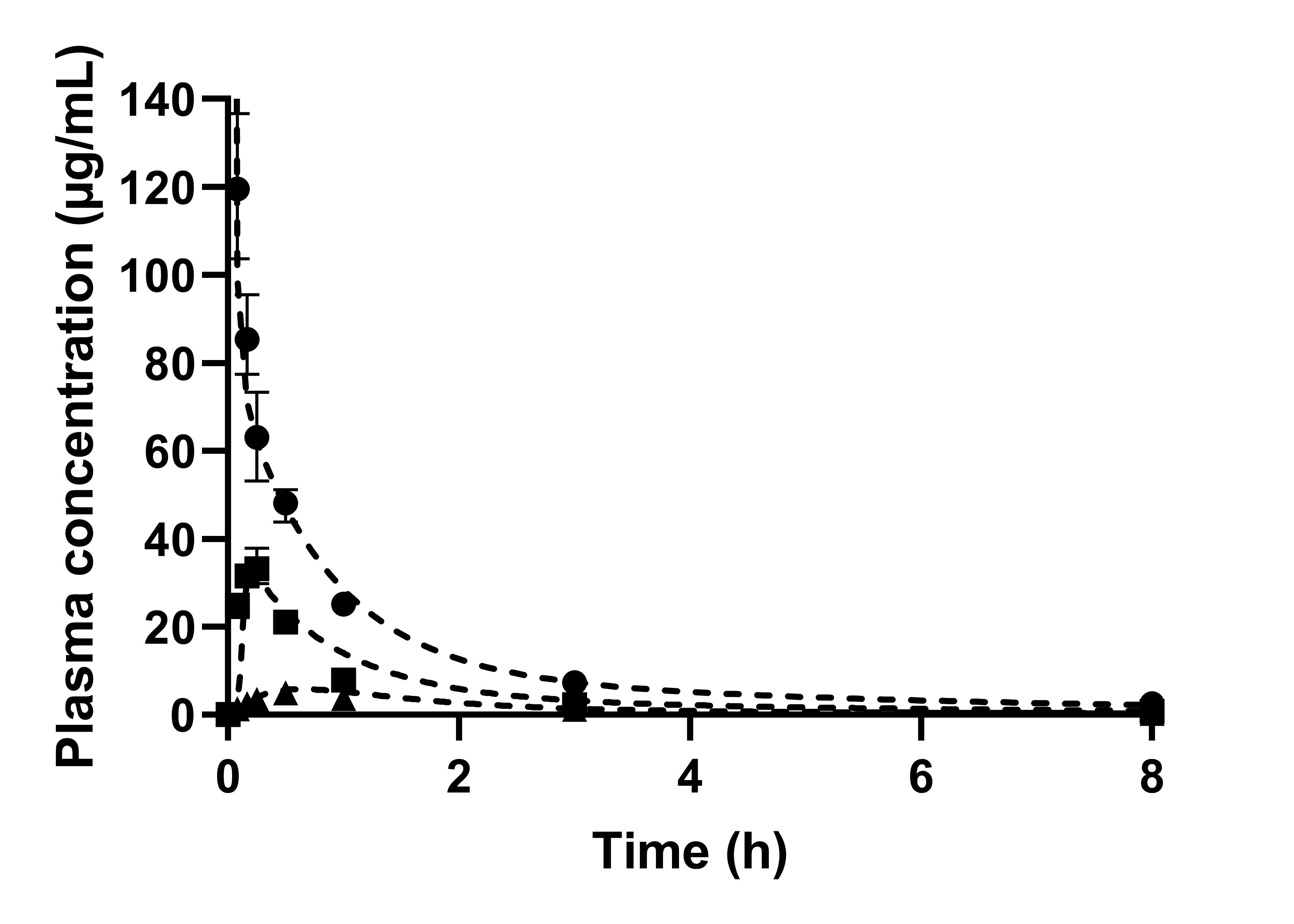
Observed and predicted plasma profiles of CorA in BALB/c mice (n=4); A: Dashed lines present the prediction. (●) show the observed data following the IV administration, (■) the observed data following the PO administration of CorA-povidone and (▲) the data following the PO administration of CorA-copovidone. Both PO administrations were administered orally as aqueous suspension.
Clinical Pharmacology - Chemical - Modeling and Simulation
Category: Poster Abstract
(W0930-11-63) Biorelevant Dissolution and PBPK Modelling to Forecast Oral Absorption – Enabling Formulations for Corallopyronin A
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- TB
Tim Becker
PhD-student
University of Bonn
Bonn, Nordrhein-Westfalen, Germany - TB
Tim Becker
PhD-student
University of Bonn
Bonn, Nordrhein-Westfalen, Germany
Presenting Author(s)
Main Author(s)
Purpose: For many novel antibiotics in the preclinical pipeline, as well as other novel compounds, sufficient oral bioavailability (BA) represents a critical parameter in preclinical drug development. Corallopyronin A (CorA), currently in preclinical drug development, is an antibiotic with activity against Gram positive and Gram negative pathogens including Wolbachia, endosymbionts of filarial nematodes2, Staphylococcus aureus and Chlamydia trachomatis. As a highly permeable, but pH dependent soluble acidic compound, the absorption of CorA (pKa 3.7; logP 5.4)1 is dependent on intestinal pH conditions. The objective of the present study was to elucidate the BA of CorA and two solubility enhanced CorA amorphous-solid-dispersions (ASD). The species mouse is often used as a preclinical efficacy model due to its lower material need, availability of various knock-out strains and an ease of handling. This makes early formulation development all the more important in order to provide a formulation with enhanced solubility and bioavailability already for preclinical animal species. Despite the high pharmacodynamic relevance of these models, oral pharmacokinetic testing remains challenging. Therefore, in vitro methods tailored to the respective species combined with physiologically-based PK-modeling (PBPK) were applied to support future formulation optimization and potentially inter species performance predictions.
Methods: In Vitro Biphasic and Monophasic Dissolution. Neat CorA and CorA-ASD formulations were investigated using the biphasic dissolution apparatus BiPHa+3. In order to mimic biorelevant conditions the experimental design was adapted to the in vivo used species mouse concerning the pH-profile, bile salt concentrations and transit time. Moreover, fed state was assumed to be the most appropriate condition. Figure 1 summarizes the experimental setup. The concentration profiles of the aqueous and organic phase were measured online continuously, with an Agilent 8454 UV-Vis spectrophotometer (Waldbronn, Germany) and quantified via external calibration curve at 394 nm. Likewise, the dissolution experiments were conducted without an organic phase to obtain dissolution parameters for PBPK modelling (data not shown).Pharmacokinetic Study. The in vivo studies were conducted in BALB/c mice. Neat CorA and CorA-ASD-formulations were administered orally as a water suspension, a CorA solution was administered intravenously, both with a dose of 36 mg/kg. The mice had free access to food over the entire experiment. Exposures (AUC0-inf) were measured and the oral bioavailability calculated. Physiological based pharmacokinetic modelling.Plasma concentration-time profiles for CorA-povidone and CorA-copovidone ASD formulations were simulated using the PBPK modelling software GastroPlus™ 9.8 (Simulations Plus, Inc., 2020). Data describing the physicochemical properties and the physiology of the investigated species were obtained from in vitro studies1 and from estimates calculated by ADMET predictor 9.5 (Simulations Plus, Inc., 2019). In the following, dissolution results were incorporated to the model, via Weibull-parameters calculated from a monophasic dissolution setup.
Results: In Vitro Biorelevant Dissolution and in vitro in vivo Relationship. The concentrations in the aqueous phase reached for neat CorA < 1% during the stomach simulation and 2% during the simulation of the small intestine. CorA povidone reached 78% followed by a decrease to 55% within approx. 30 minutes. For CorA-copovidone only 10% were achieved by the end of the dissolution. By using the ASD formulation approach in vitro biphasic dissolution exhibited enhanced dissolution performances. The amount of partitioned drug into the organic phase after 80 min represents the in vitro measured fraction absorbed. Neat CorA achieved only 4% partitioning, whereas for CorA-povidone 34% and for CorA-copovidone 13% could be achieved, corroborating the solubility enhancement by the formulation principle. The end point concentrations of the organic phase were correlated with the bioavailability of neat CorA and the CorA-ASD-formulations. A good correlation between both parameters was determined (R2 = 0.9966, Fig. 2). Physiological based pharmacokinetic modelling. The IV data were used to provide elimination and distribution behavior. Measured and predicted physicochemical and physiological properties were used to build a PBPK model for oral administration of CorA-ASD formulations. By incorporating the dissolution profiles of the monophasic dissolutions, plasma concentrations of the CorA-ASD-formulations were successfully predicted. Prediction errors (PE) < 15% were reached for CorA-povidone (Cmax: 1.71%; AUC0-inf: -11.31%). For CorA-copovidone Cmax and AUC0-inf were slightly overpredicted (Cmax: -16.20%; AUC0-inf: -12.43%), but still within an acceptable range (Fig. 3).
Conclusion: The mini-scale biphasic dissolution test BiPHa+ adjusted to gastric pH-levels of mice resulted in level C in vitro in vivo relationship. Moreover, determined intrinsic dissolution data in biorelevant media enabled to deduct dissolution as the decisive mechanism governing oral bioavailability of CorA and CorA-formulations. Hence, the presented setup confirmed its in vivo relevance for mice, thus providing a material saving method to optimize oral treatment of mice even in early preclinical studies. The incorporation of biorelevant dissolution into the PBPK model allowed a prediction of plasma concentration in mice. Additionally, the method can potentially be used to forecast the performance of CorA in other species via changed pH-settings of the BiPHa+ assay combined with PBPK-modelling to identify the later dose regimen for human.
References: 1. Krome, A. K. et al. Solubility and Stability Enhanced Oral Formulations for the Anti-Infective Corallopyronin A. Pharmaceutics 21, (2020).
2. Schiefer, A. et al. Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections. PLoS Negl Trop Dis 14, (2020).
3. Denninger, A., et al. A Rational Design of a Biphasic Dissolution Setup—Modelling of Biorelevant Kinetics for a Ritonavir Hot-Melt Extruded Amorphous Solid Dispersion. Pharmaceutics 12, 237 (2020).
Acknowledgements:We would like to thank the German Center for Infection Research (www.DZIF.de) grant numbers TTU 09.807, 09.816, 09.914 and the Federal Ministry of Education and Research (www.BMBF.de) grant number 16GW0229 for funding.

Biphasic dissolution setup for the mouse model.

A: Biphasic dissolution under mouse gut conditions; B: Level C in vitro in vivo relationship: In vitro partitioned drug into the organic phase and in vivo BA; neat CorA (●), CorA-povidone (■) and CorA-copovidone (▲).

Observed and predicted plasma profiles of CorA in BALB/c mice (n=4); A: Dashed lines present the prediction. (●) show the observed data following the IV administration, (■) the observed data following the PO administration of CorA-povidone and (▲) the data following the PO administration of CorA-copovidone. Both PO administrations were administered orally as aqueous suspension.
