Back
Purpose: Although hydrophobic compounds can be more easily loaded into nanoparticles by the conventional o/w emulsion method, the encapsulation of hydrophilic items into hydrophobic nanosized matrices is difficult. Hydrophilic molecules are generally encapsulated using a double emulsion method. One frequent disadvantage of this strategy is low loading capacity. Unlike the bulk materials, the nanoscale material physical properties can vary with particle size. A hypothesis is that hydrophilic particles can show a hydrophobic properties at a small particle size. [1] Based on this hypothesis, a new method that could achieve a higher loading capacity was developed to encapsulate hydrophilic molecules in a hydrophobic polymer. Trivalent arsenic salt is considered as an ideal hydrophilic model compound for testing this new method. Arsenic trioxide (ATO), which has been used for medicinal applications for more than a thousand years in traditional Chinese and Indian medicine. In modern medicine, it also has been shown to inhibit the development of cancer, for example, leukemia.[2] For many of the anti-cancer drugs, preparing it into nanoparticles can reduce its severe toxicity and side effects. As a poor water and organic solvent soluble compound, ATO can be dissolved in alkali media to form soluble trivalent arsenic salt. However, this hydrophilic property makes it difficult to be loaded into nanoparticle compared to other hydrophobic drugs. In our study, trivalent arsenic salt was encapsulated into nanoparticles through this new strategy.
Methods: Hydrophobic polymer nanoparticles containing trivalent arsenic salt were prepared by the oil in water (O/W) emulsion method. Different from the conventional O/W method that drug is dissolved in the solvent, trivalent arsenic salt nanosuspension was incorporated into the oil phase. The W/O emulsion method was used to prepare the trivalent arsenic salt nanosuspension. The trivalent arsenic salt solution was in the water phase and polymer in the organic phase. When the interface of two liquids is sonicated, an emulsion is formed. After the solvents in emulsion was evaporated, even-sized hydrophilic trivalent arsenic salt particles were dispersed in the porous structure which formed by the hydrophobic polymer. The organic solvent was added to dissolve the porous polymer. The hydrophilic salt particles were kept and formed the nanosuspension. This nanosuspension can be directly incorporated into oil phase. In the final step, nanoparticles were prepared by the O/W method with the solvent evaporation. Nanoparticle samples were dried by lyophilization. Scanning electron microscope (SEM) was used to study the size distribution and morphology of the nanoparticles. Dynamic light scattering (DLS) was used to study the size distribution of nanosuspension in acetonitrile/water miscible which mimics the W/O emulsion method’s condition during the nanoparticle preparation. The loading capacity of the nanoparticles was measured by inductively coupled plasma mass spectrometry (ICP-MS).
Results: Trivalent arsenic salt-loaded PLGA-PEG nanoparticles were obtained. Two repeated batches of pre-prepared nanosuspension’s size distribution are 267.9±9.5 nm (batch A) and 285.6±15.1 nm (batch B), respectively (Figure 1). Trivalent arsenic salt percent loading of nanosuspension with O/W method were 6.33% (batch A) and 4.95% (batch B), respectively, which is higher than the conventional W/O/W method (0.019%) and O/W (0.369%) method loading arsenic salt solution. The nanosuspension dynamic distribution of different particles sizes in mimic nanoparticle preparation process was measured. The volume percentage of large particles (larger than 250 nm) decreased and small particles (around 70 nm) increased (Figure 2). The dynamic process of average particle size changing also was measured. The average particle size of trivalent arsenic salt in water/organic miscible solvent nanosuspension decreased over time and trended to a stable value after a 50 min. period (Figure 3). These two results, illustrate that larger particles dissolved or split into smaller particles in the water/organic mixture, and trended to a steady state particle size. Because the dynamic size distribution was measured under a relatively static condition, this process should be slower than the ultrasonic and stirring condition during the real nanoparticle preparation. During the O/W nanoparticle preparation process, the organic solvent formed monodisperses droplets, which containing dissolved “payload” and polymer. Then the organic solvent is evaporated from the system causing the polymer to precipitate within the “payload” forming solid particles. The hydrophobic “payload” is easier to stay in the organic solvent droplet with the polymer than form the nanoparticle with a high loading capacity. In our study, size distribution of nanosuspension influences the loading capacity of trivalent arsenic salt in nanoparticles. Nanosuspension with smaller particle size achieved a higher loading capacity which supports the hypothesis that small size hydrophilic materials’ particles can show a hydrophobic property.
Conclusion: This study to the best of the authors’ knowledge describes for the first time a new strategy that has a high loading capacity to encapsulate hydrophilic molecules rather than using the conventional W/O/W methods. Such a method has great potential for hydrophilic compound loading and delivery.
References: 1. Chiu, C.C., et al., Size-dependent hydrophobic to hydrophilic transition for nanoparticles: a molecular dynamics study. J Chem Phys, 2009. 131(24): p. 244706.
2. Glienke, W., et al., Down-regulation of wt1 expression in leukemia cell lines as part of apoptotic effect in arsenic treatment using two compounds. Leuk Lymphoma, 2006. 47(8): p. 1629-38.
Acknowledgements:NoStopharm Inc. and Maryland Industrial Partnerships (MIPS) for technical assistance and financial support.
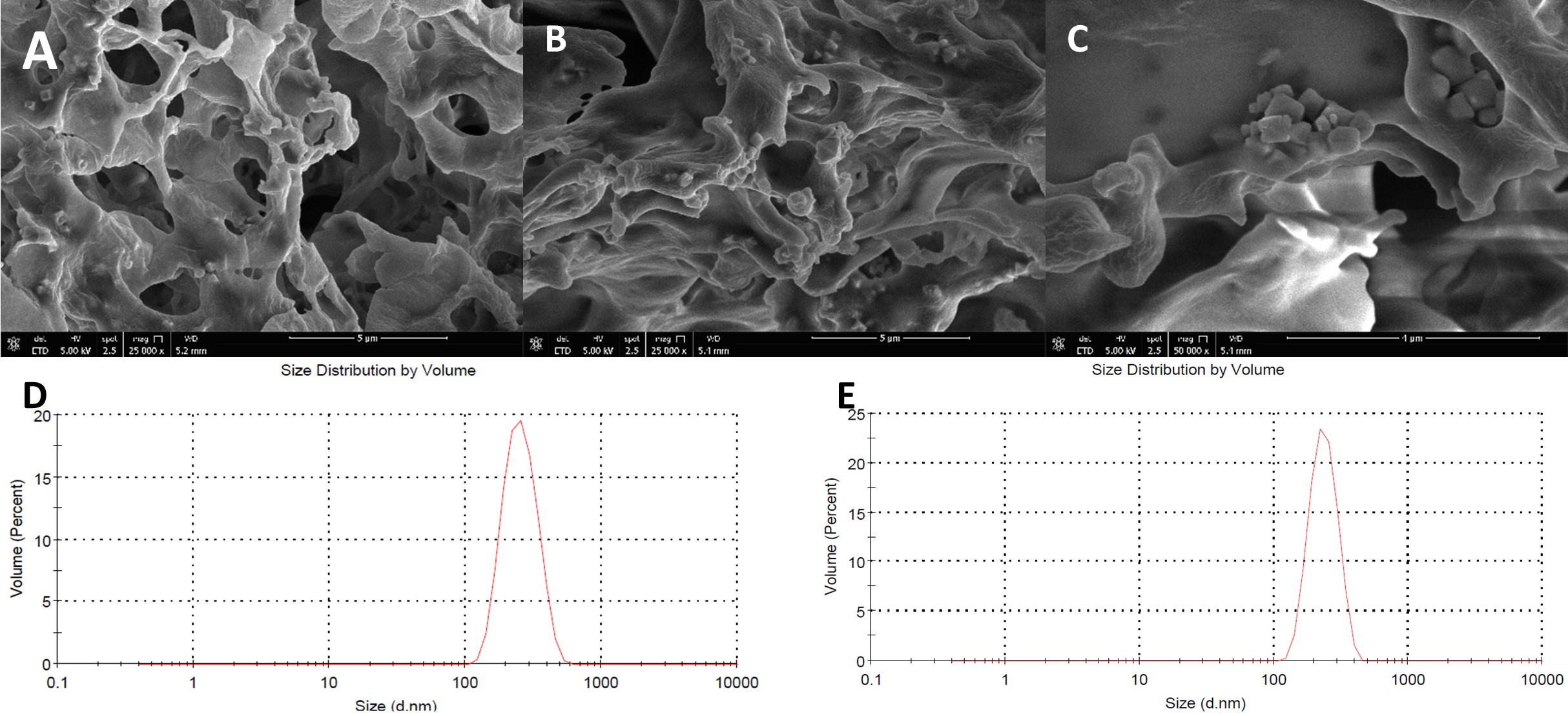
Figure. 1. Particle size distribution of nanosuspension. (A) TEM image of batch A nanosuspension; (B) TEM image of batch B nanosuspension low magnification (C) TEM image of batch B nanosuspension high magnification; (D) DLS spectrum of batch A nanosuspension; (E) DLS spectrum of batch B nanosuspension
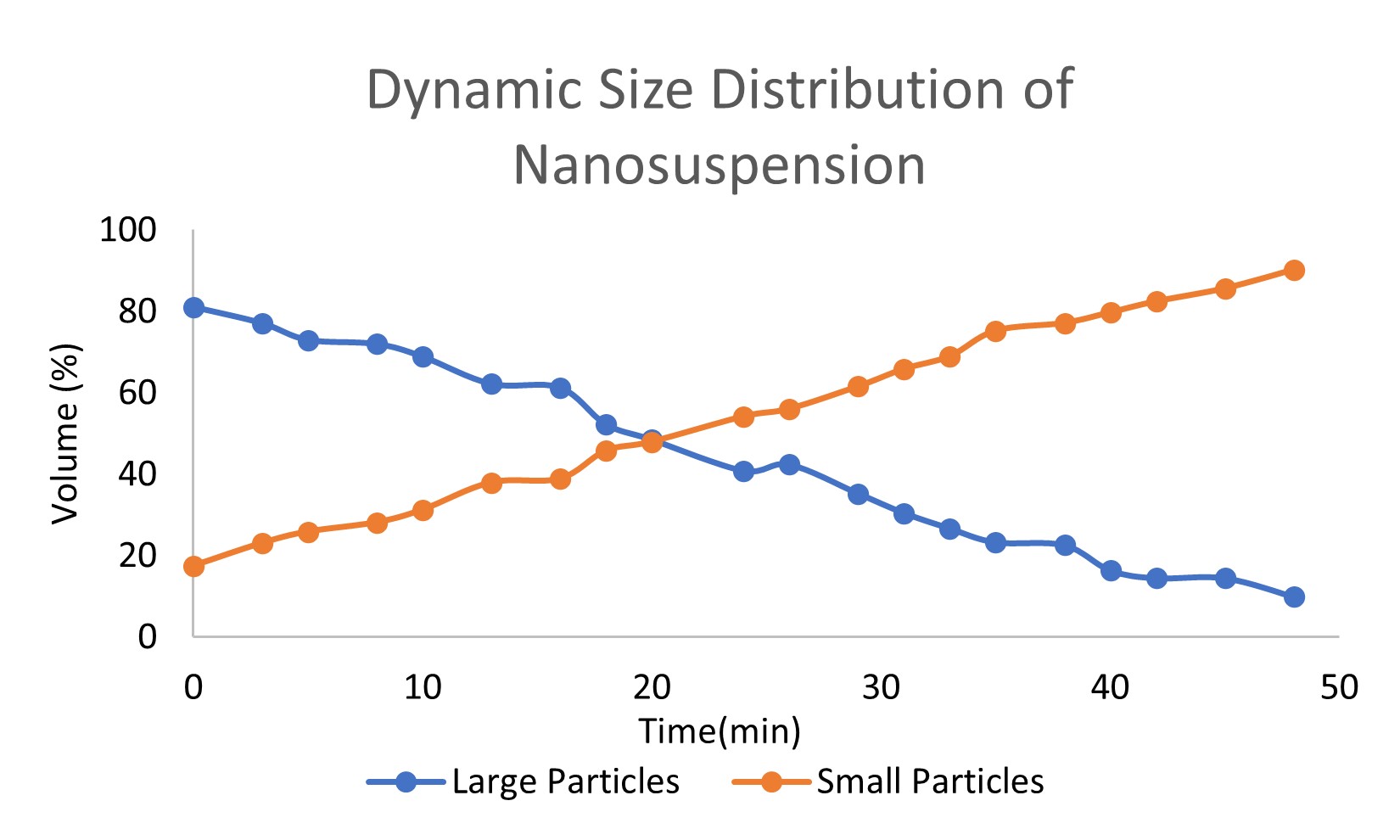
Figure. 2. Dynamic size distribution of nanosuspension
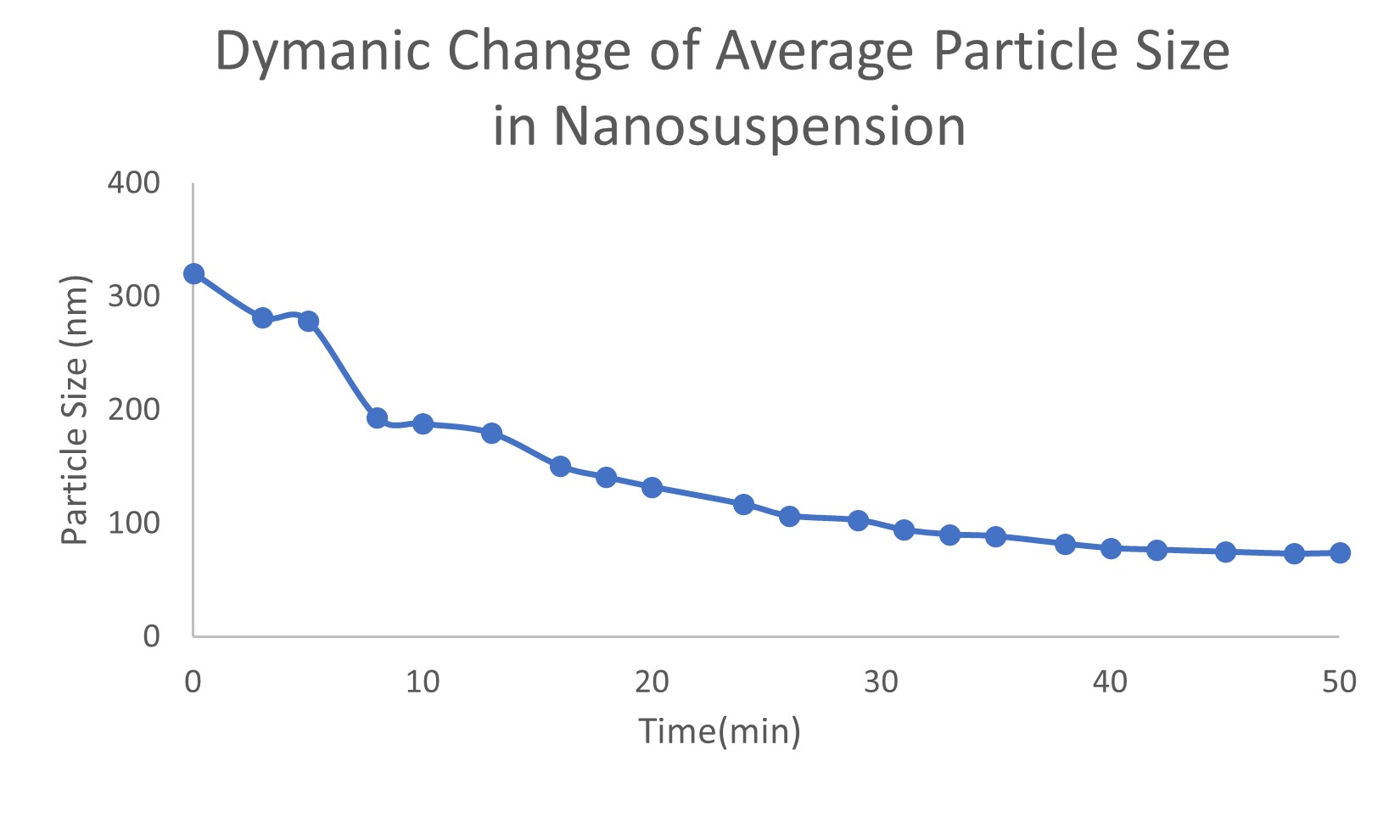
Figure. 3. Dynamic change of average particle size in nanosuspension
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W0930-07-41) Nanosuspension Organic Phase Instead of Solution Water Phase: A New Approach to Loading Hydrophilic Trivalent Arsenic Salt with Hydrophobic Polymer
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- YW
Yihan Wang, MS
University of Maryland Baltimore
Baltimore, Maryland, United States - YW
Yihan Wang, MS
University of Maryland Baltimore
Baltimore, Maryland, United States
Presenting Author(s)
Main Author(s)
Purpose: Although hydrophobic compounds can be more easily loaded into nanoparticles by the conventional o/w emulsion method, the encapsulation of hydrophilic items into hydrophobic nanosized matrices is difficult. Hydrophilic molecules are generally encapsulated using a double emulsion method. One frequent disadvantage of this strategy is low loading capacity. Unlike the bulk materials, the nanoscale material physical properties can vary with particle size. A hypothesis is that hydrophilic particles can show a hydrophobic properties at a small particle size. [1] Based on this hypothesis, a new method that could achieve a higher loading capacity was developed to encapsulate hydrophilic molecules in a hydrophobic polymer. Trivalent arsenic salt is considered as an ideal hydrophilic model compound for testing this new method. Arsenic trioxide (ATO), which has been used for medicinal applications for more than a thousand years in traditional Chinese and Indian medicine. In modern medicine, it also has been shown to inhibit the development of cancer, for example, leukemia.[2] For many of the anti-cancer drugs, preparing it into nanoparticles can reduce its severe toxicity and side effects. As a poor water and organic solvent soluble compound, ATO can be dissolved in alkali media to form soluble trivalent arsenic salt. However, this hydrophilic property makes it difficult to be loaded into nanoparticle compared to other hydrophobic drugs. In our study, trivalent arsenic salt was encapsulated into nanoparticles through this new strategy.
Methods: Hydrophobic polymer nanoparticles containing trivalent arsenic salt were prepared by the oil in water (O/W) emulsion method. Different from the conventional O/W method that drug is dissolved in the solvent, trivalent arsenic salt nanosuspension was incorporated into the oil phase. The W/O emulsion method was used to prepare the trivalent arsenic salt nanosuspension. The trivalent arsenic salt solution was in the water phase and polymer in the organic phase. When the interface of two liquids is sonicated, an emulsion is formed. After the solvents in emulsion was evaporated, even-sized hydrophilic trivalent arsenic salt particles were dispersed in the porous structure which formed by the hydrophobic polymer. The organic solvent was added to dissolve the porous polymer. The hydrophilic salt particles were kept and formed the nanosuspension. This nanosuspension can be directly incorporated into oil phase. In the final step, nanoparticles were prepared by the O/W method with the solvent evaporation. Nanoparticle samples were dried by lyophilization. Scanning electron microscope (SEM) was used to study the size distribution and morphology of the nanoparticles. Dynamic light scattering (DLS) was used to study the size distribution of nanosuspension in acetonitrile/water miscible which mimics the W/O emulsion method’s condition during the nanoparticle preparation. The loading capacity of the nanoparticles was measured by inductively coupled plasma mass spectrometry (ICP-MS).
Results: Trivalent arsenic salt-loaded PLGA-PEG nanoparticles were obtained. Two repeated batches of pre-prepared nanosuspension’s size distribution are 267.9±9.5 nm (batch A) and 285.6±15.1 nm (batch B), respectively (Figure 1). Trivalent arsenic salt percent loading of nanosuspension with O/W method were 6.33% (batch A) and 4.95% (batch B), respectively, which is higher than the conventional W/O/W method (0.019%) and O/W (0.369%) method loading arsenic salt solution. The nanosuspension dynamic distribution of different particles sizes in mimic nanoparticle preparation process was measured. The volume percentage of large particles (larger than 250 nm) decreased and small particles (around 70 nm) increased (Figure 2). The dynamic process of average particle size changing also was measured. The average particle size of trivalent arsenic salt in water/organic miscible solvent nanosuspension decreased over time and trended to a stable value after a 50 min. period (Figure 3). These two results, illustrate that larger particles dissolved or split into smaller particles in the water/organic mixture, and trended to a steady state particle size. Because the dynamic size distribution was measured under a relatively static condition, this process should be slower than the ultrasonic and stirring condition during the real nanoparticle preparation. During the O/W nanoparticle preparation process, the organic solvent formed monodisperses droplets, which containing dissolved “payload” and polymer. Then the organic solvent is evaporated from the system causing the polymer to precipitate within the “payload” forming solid particles. The hydrophobic “payload” is easier to stay in the organic solvent droplet with the polymer than form the nanoparticle with a high loading capacity. In our study, size distribution of nanosuspension influences the loading capacity of trivalent arsenic salt in nanoparticles. Nanosuspension with smaller particle size achieved a higher loading capacity which supports the hypothesis that small size hydrophilic materials’ particles can show a hydrophobic property.
Conclusion: This study to the best of the authors’ knowledge describes for the first time a new strategy that has a high loading capacity to encapsulate hydrophilic molecules rather than using the conventional W/O/W methods. Such a method has great potential for hydrophilic compound loading and delivery.
References: 1. Chiu, C.C., et al., Size-dependent hydrophobic to hydrophilic transition for nanoparticles: a molecular dynamics study. J Chem Phys, 2009. 131(24): p. 244706.
2. Glienke, W., et al., Down-regulation of wt1 expression in leukemia cell lines as part of apoptotic effect in arsenic treatment using two compounds. Leuk Lymphoma, 2006. 47(8): p. 1629-38.
Acknowledgements:NoStopharm Inc. and Maryland Industrial Partnerships (MIPS) for technical assistance and financial support.

Figure. 1. Particle size distribution of nanosuspension. (A) TEM image of batch A nanosuspension; (B) TEM image of batch B nanosuspension low magnification (C) TEM image of batch B nanosuspension high magnification; (D) DLS spectrum of batch A nanosuspension; (E) DLS spectrum of batch B nanosuspension

Figure. 2. Dynamic size distribution of nanosuspension

Figure. 3. Dynamic change of average particle size in nanosuspension
