Back
Purpose: Niclosamide is a poorly water-soluble molecule that has recently been tested as a chemotherapeutic, antibacterial, broad-spectrum antiviral, among other therapeutic indications. Recently, our group developed a niclosamide amorphous solid dispersion (ASD) that generates nanoparticles during its dissolution, increasing niclosamide’s apparent solubility by more than 60-fold (i.e. from 6.6 ± 0.4 to 481.7 ± 22.2 µg/mL) and also its bioavailability in vivo.1 Unfortunately, the formulation undergoes recrystallization in acidic media conditions. In general, there is a lack of literature addressing the development of enteric capsule/tablet formulations for an ASD with these characteristics. In this work, we aimed to understand further the solid-state properties of the nanoparticles, to select a proper enteric dosage form and to conduct in vivo testing in beagle dogs.
Methods: Niclosamide ASD is manufactured by hot-melt extrusion and comprises niclosamide anhydrate, copovidone, and vitamin E TPGS at a ratio of 35/60/5. The resulting extrudate was milled using a Fitzmill to obtain granules of niclosamide ASD. Granules of niclosamide ASD, tablets, and capsules underwent dissolution testing using a USP type II apparatus with low volume vessels at 37.0 ± 0.5 °C and a paddle speed of 100 rpm. The pH-shift dissolution tests were conducted in 120 mL of 0.1N HCl for 2h, after which the tablets/capsules were transferred to 150 mL of fasted state simulated intestinal fluid (FaSSIF) medium. For characterization of the nanoparticles released from the dosage forms during dissolution testing, a sample from the vessel was collected using 0.2 µm polyethersulfone filters after 2h of dissolution in FaSSIF. 5 mL were collected and added into a Float-A-Lyzer® Device (Repligen, CA, USA) for dialysis. The sample was dialyzed using 1 L of water supersaturated with niclosamide (added in excess) for 24h. This was done in order to remove the excess salts from the sample. These liquid samples were vitrified and observed using a Glacios Cryo-TEM with Falcon 4. In addition, dialyzed samples were frozen using liquid nitrogen and lyophilized to determine their solid-state morphology using Wide Angle X-ray Scattering (WAXS). In the development of an oral dosage form, niclosamide ASD granules were encapsulated in two commercially available enteric capsules, Capsuline® (HPMCP/HPMC) and EudracapTM enteric (HPMC/Eudragit® polymers). In addition, enteric-coated tablets were also prepared. The tablet cores were sequentially coated with Opadry II Clear for seal coating (3% weight gain) followed by Acryl-EZE® 93 A at a 10% weight gain to provide enteric protection. In the acid uptake test, six capsules/tablets were weighed and placed into a disintegration test system containing 0.1N HCl at 37°C for 2h. After the test, the capsules/tablets were surface dried and weighed again to determine the degree of weight increase. In vivo testing in beagle dogs following oral administration of tablets containing niclosamide ASD at a dose of 75 mg/kg was performed. Blood samples were processed into plasma and analyzed by HPLC/MS.
Results: Cryo-TEM revealed that the niclosamide ASD generates spherical nanoparticles with diameters of approximately 150 nm during dissolution in FaSSIF medium (Figure 1A). In addition, these particles were covered by a bilayer membrane corona of about 4.5 nm likely due to the presence of bile salts in the dissolution medium. WAXS confirmed that the nanoparticles were amorphous (Figure 1B). As reported previously, niclosamide ASD undergoes recrystallization in acidic media.1 Oral dosage forms containing niclosamide ASD were tested in pH dissolution and acid-uptake tests (Figure 2). The capsules showed higher percentages of weight gain and, unfortunately, even ruptured during the acid uptake test. The pH-shift dissolution test confirmed that tablets are a more reliable enteric dosage form for the niclosamide ASD. These tablets were administered to beagle dogs, and plasma concentrations of up to 149 ± 79.2 ng/mL were detected at an administered dose of 75 mg/kg niclosamide (Figure 3). These plasma concentrations of niclosamide were higher than those reported in the literature using solubilized niclosamide at a higher dose (100 mg/kg).2
Conclusion: Niclosamide ASD generates amorphous nanoparticles during dissolution. It was possible to formulate an enteric oral dosage form of niclosamide ASD without hindering the generation of nanoparticles and maintaining the increase of niclosamide’s apparent solubility. The enteric-coated tablets successfully increased niclosamide plasma levels in dogs.
References: 1. Jara, M. O., Warnken, Z. N. & Williams, R. O. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 13, 97 (2021).
2. Choi, H.-I. et al. Bioanalysis of niclosamide in plasma using liquid chromatography-tandem mass and application to pharmacokinetics in rats and dogs. Journal of Chromatography B 1179, 122862 (2021).
Acknowledgements:
Funding: This research on viral infections (including COVID-19) was funded by TFF Pharmaceuticals, Inc. through a sponsored research agreement with the University of Texas at Austin. Warnken is partially supported by this sponsored research agreement with TFF Pharmaceuticals Inc. Miguel O. Jara acknowledges the funding support from the Equal Opportunities Fulbright—CONICYT Scholarship 56170009.
Conflicts of Interest: Jara, Warnken, Sahakijpiarn and Williams are co-inventors on IP related to this paper. The University of Texas System has licensed this IP to TFF Pharmaceuticals, Inc. Moon and Sahakijpijarn acknowledge consulting for TFF Pharmaceuticals, Inc. Williams owns equity in and consults for TFF Pharmaceuticals, Inc.
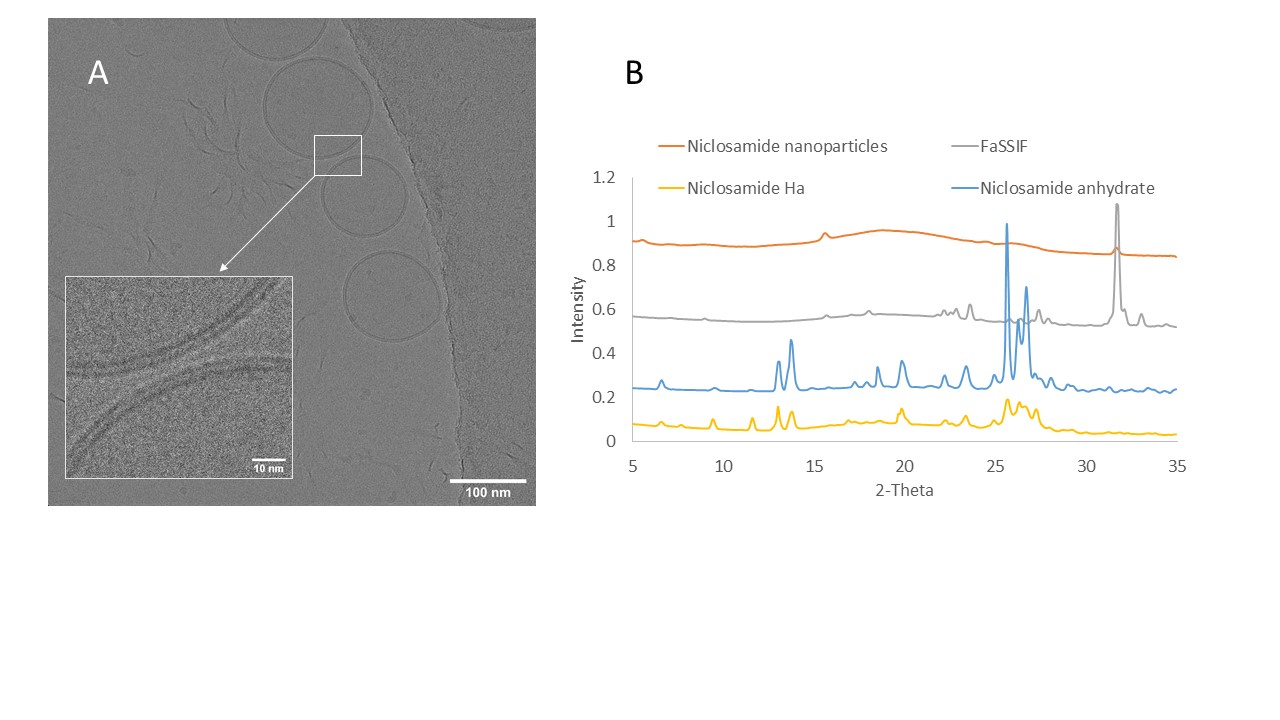
Figure 1. (A) Cryo-TEM of niclosamide ASD nanoparticles after 24 h of dialysis in niclosamide-supersaturated water (5 mL of the sample over 1 L of water) to remove excess salts. Even after the 24h dialysis, these particles were covered by a bilayer membrane corona of 4.5 nm. B) Same dialyzed samples were lyophilized and analyzed using Wide-Angle X-ray Scattering (WAXS), confirming that the nanoparticles were amorphous.
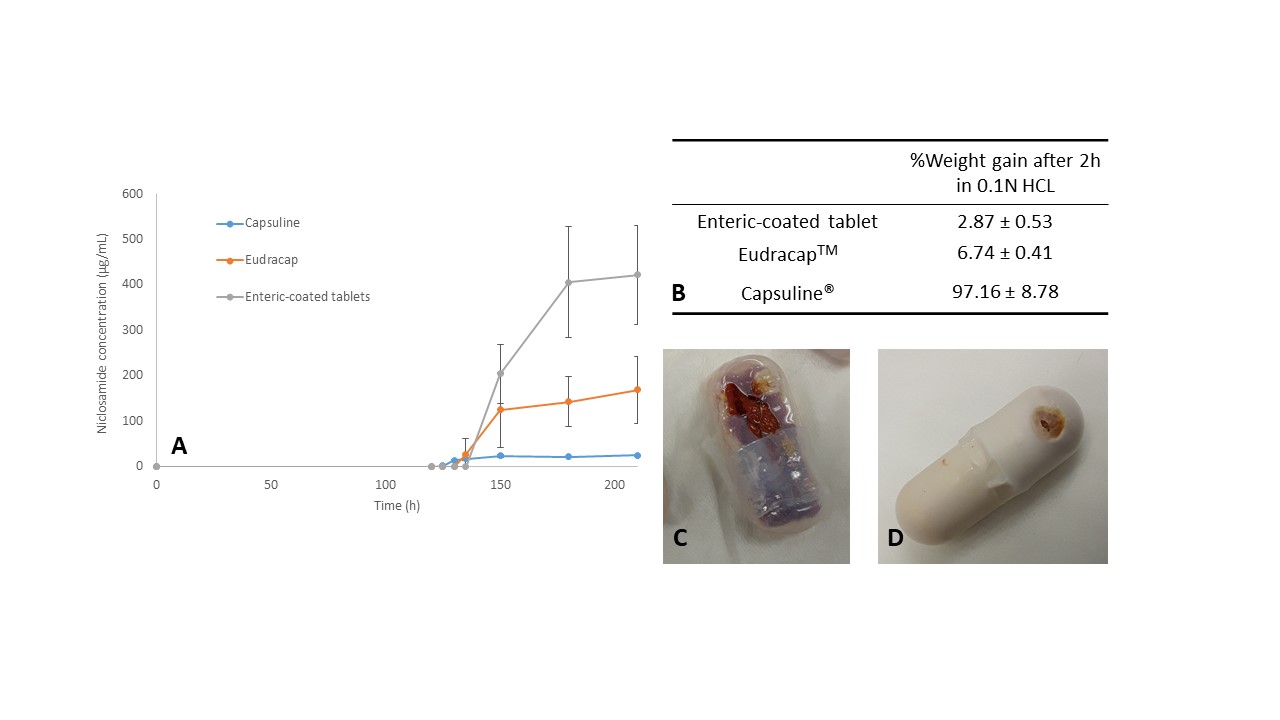
Figure 2. (A) pH-shift dissolution test of enteric capsules and enteric-coated capsules. (B) Acid uptake test and weight gain of enteric capsules and enteric-coated tablets after 2h in 0.1N HCl. (C) Capsuline® capsules after the acid uptake test. (D) Some Eudracap capsules ruptured during the test. However, this was not observed during dissolution tests (USP type II apparatus).
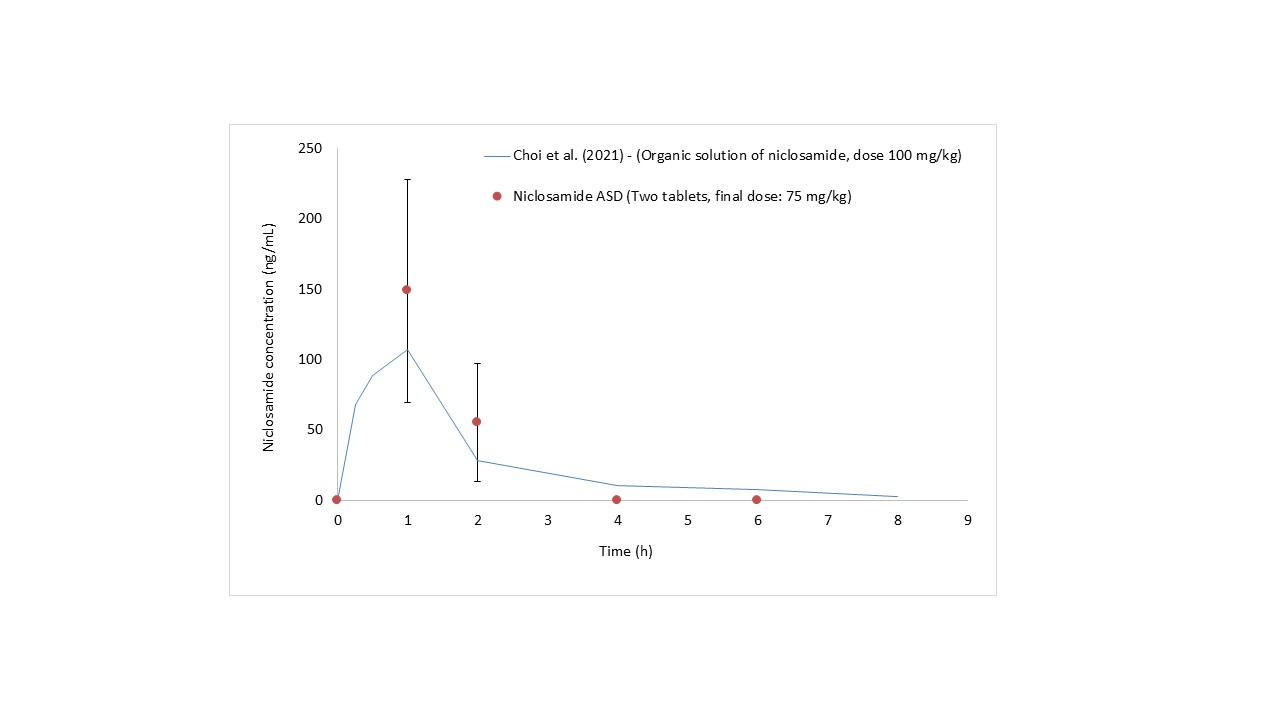
Figure 3. Plasma concentrations of niclosamide in Beagle dogs after administering tablets containing niclosamide ASD. The data from Choi et al. was added to the chart as a comparison. In that article, niclosamide was dissolved in a mixture containing solvents, polymers, and pH modifiers (DMSO, PEG, NaOH, etc). The data was extracted from the publication using the software PlotDigitizer. In our method, after 4h, the plasma levels were zero because they were below the limit of quantification of 40 ng/mL Reference: Choi, H.-I. et al. Bioanalysis of niclosamide in plasma using liquid chromatography-tandem mass and application to pharmacokinetics in rats and dogs. Journal of Chromatography B 1179, 122862 (2021)
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W0930-12-68) Development and In Vivo Testing of an Enteric Oral Dosage Form of an Amorphous Solid Dispersion in Beagle Dogs that Generates Amorphous Nanoparticles during Dissolution
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- MJ
Miguel O. Jara Gonzalez
University of Texas at Austin
Austin, Texas, United States - MJ
Miguel O. Jara Gonzalez
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: Niclosamide is a poorly water-soluble molecule that has recently been tested as a chemotherapeutic, antibacterial, broad-spectrum antiviral, among other therapeutic indications. Recently, our group developed a niclosamide amorphous solid dispersion (ASD) that generates nanoparticles during its dissolution, increasing niclosamide’s apparent solubility by more than 60-fold (i.e. from 6.6 ± 0.4 to 481.7 ± 22.2 µg/mL) and also its bioavailability in vivo.1 Unfortunately, the formulation undergoes recrystallization in acidic media conditions. In general, there is a lack of literature addressing the development of enteric capsule/tablet formulations for an ASD with these characteristics. In this work, we aimed to understand further the solid-state properties of the nanoparticles, to select a proper enteric dosage form and to conduct in vivo testing in beagle dogs.
Methods: Niclosamide ASD is manufactured by hot-melt extrusion and comprises niclosamide anhydrate, copovidone, and vitamin E TPGS at a ratio of 35/60/5. The resulting extrudate was milled using a Fitzmill to obtain granules of niclosamide ASD. Granules of niclosamide ASD, tablets, and capsules underwent dissolution testing using a USP type II apparatus with low volume vessels at 37.0 ± 0.5 °C and a paddle speed of 100 rpm. The pH-shift dissolution tests were conducted in 120 mL of 0.1N HCl for 2h, after which the tablets/capsules were transferred to 150 mL of fasted state simulated intestinal fluid (FaSSIF) medium. For characterization of the nanoparticles released from the dosage forms during dissolution testing, a sample from the vessel was collected using 0.2 µm polyethersulfone filters after 2h of dissolution in FaSSIF. 5 mL were collected and added into a Float-A-Lyzer® Device (Repligen, CA, USA) for dialysis. The sample was dialyzed using 1 L of water supersaturated with niclosamide (added in excess) for 24h. This was done in order to remove the excess salts from the sample. These liquid samples were vitrified and observed using a Glacios Cryo-TEM with Falcon 4. In addition, dialyzed samples were frozen using liquid nitrogen and lyophilized to determine their solid-state morphology using Wide Angle X-ray Scattering (WAXS). In the development of an oral dosage form, niclosamide ASD granules were encapsulated in two commercially available enteric capsules, Capsuline® (HPMCP/HPMC) and EudracapTM enteric (HPMC/Eudragit® polymers). In addition, enteric-coated tablets were also prepared. The tablet cores were sequentially coated with Opadry II Clear for seal coating (3% weight gain) followed by Acryl-EZE® 93 A at a 10% weight gain to provide enteric protection. In the acid uptake test, six capsules/tablets were weighed and placed into a disintegration test system containing 0.1N HCl at 37°C for 2h. After the test, the capsules/tablets were surface dried and weighed again to determine the degree of weight increase. In vivo testing in beagle dogs following oral administration of tablets containing niclosamide ASD at a dose of 75 mg/kg was performed. Blood samples were processed into plasma and analyzed by HPLC/MS.
Results: Cryo-TEM revealed that the niclosamide ASD generates spherical nanoparticles with diameters of approximately 150 nm during dissolution in FaSSIF medium (Figure 1A). In addition, these particles were covered by a bilayer membrane corona of about 4.5 nm likely due to the presence of bile salts in the dissolution medium. WAXS confirmed that the nanoparticles were amorphous (Figure 1B). As reported previously, niclosamide ASD undergoes recrystallization in acidic media.1 Oral dosage forms containing niclosamide ASD were tested in pH dissolution and acid-uptake tests (Figure 2). The capsules showed higher percentages of weight gain and, unfortunately, even ruptured during the acid uptake test. The pH-shift dissolution test confirmed that tablets are a more reliable enteric dosage form for the niclosamide ASD. These tablets were administered to beagle dogs, and plasma concentrations of up to 149 ± 79.2 ng/mL were detected at an administered dose of 75 mg/kg niclosamide (Figure 3). These plasma concentrations of niclosamide were higher than those reported in the literature using solubilized niclosamide at a higher dose (100 mg/kg).2
Conclusion: Niclosamide ASD generates amorphous nanoparticles during dissolution. It was possible to formulate an enteric oral dosage form of niclosamide ASD without hindering the generation of nanoparticles and maintaining the increase of niclosamide’s apparent solubility. The enteric-coated tablets successfully increased niclosamide plasma levels in dogs.
References: 1. Jara, M. O., Warnken, Z. N. & Williams, R. O. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 13, 97 (2021).
2. Choi, H.-I. et al. Bioanalysis of niclosamide in plasma using liquid chromatography-tandem mass and application to pharmacokinetics in rats and dogs. Journal of Chromatography B 1179, 122862 (2021).
Acknowledgements:
Funding: This research on viral infections (including COVID-19) was funded by TFF Pharmaceuticals, Inc. through a sponsored research agreement with the University of Texas at Austin. Warnken is partially supported by this sponsored research agreement with TFF Pharmaceuticals Inc. Miguel O. Jara acknowledges the funding support from the Equal Opportunities Fulbright—CONICYT Scholarship 56170009.
Conflicts of Interest: Jara, Warnken, Sahakijpiarn and Williams are co-inventors on IP related to this paper. The University of Texas System has licensed this IP to TFF Pharmaceuticals, Inc. Moon and Sahakijpijarn acknowledge consulting for TFF Pharmaceuticals, Inc. Williams owns equity in and consults for TFF Pharmaceuticals, Inc.

Figure 1. (A) Cryo-TEM of niclosamide ASD nanoparticles after 24 h of dialysis in niclosamide-supersaturated water (5 mL of the sample over 1 L of water) to remove excess salts. Even after the 24h dialysis, these particles were covered by a bilayer membrane corona of 4.5 nm. B) Same dialyzed samples were lyophilized and analyzed using Wide-Angle X-ray Scattering (WAXS), confirming that the nanoparticles were amorphous.

Figure 2. (A) pH-shift dissolution test of enteric capsules and enteric-coated capsules. (B) Acid uptake test and weight gain of enteric capsules and enteric-coated tablets after 2h in 0.1N HCl. (C) Capsuline® capsules after the acid uptake test. (D) Some Eudracap capsules ruptured during the test. However, this was not observed during dissolution tests (USP type II apparatus).

Figure 3. Plasma concentrations of niclosamide in Beagle dogs after administering tablets containing niclosamide ASD. The data from Choi et al. was added to the chart as a comparison. In that article, niclosamide was dissolved in a mixture containing solvents, polymers, and pH modifiers (DMSO, PEG, NaOH, etc). The data was extracted from the publication using the software PlotDigitizer. In our method, after 4h, the plasma levels were zero because they were below the limit of quantification of 40 ng/mL Reference: Choi, H.-I. et al. Bioanalysis of niclosamide in plasma using liquid chromatography-tandem mass and application to pharmacokinetics in rats and dogs. Journal of Chromatography B 1179, 122862 (2021)
